Introduction: The “magic bullet”
Paul Ehrlich (1854-1915), a German physician-scientist, was a man with ingenious insights. Renowned as the founder of immunology and chemotherapy, his groundbreaking scientific contributions earned him a Noble Prize in Physiology and Medicine in 1908. His legacy is honoured to this day, with the prestigious German Paul Ehrlich Institute being named after him.
By theorising the existence of receptors, proteins that respond to specific chemical signals, Ehrlich envisioned the potential of “magic bullets” in medicine in 1913. These are drugs that directly attack the intended target by aiming at its specific receptors. As a result, healthy cells are spared from harm, limiting the risk of adverse side effects to the patient. The principle, “wir müssen chemisch zielen lernen,” i.e., “we must learn to aim chemically,” was, indeed, introduced by Ehrlich.
Notwithstanding Ehrlich’s achievements, his initial research into immunogenic receptors unique to cancer cells was not fruitful. Nevertheless, those who continued his research in the subsequent decades eventually succeeded in identifying specific receptors on cancer cell surfaces, laying the groundwork for the innovation of antibody-drug conjugates (ADCs) in the 21st century.
Principle of Antibody-drug conjugates (ADCs)
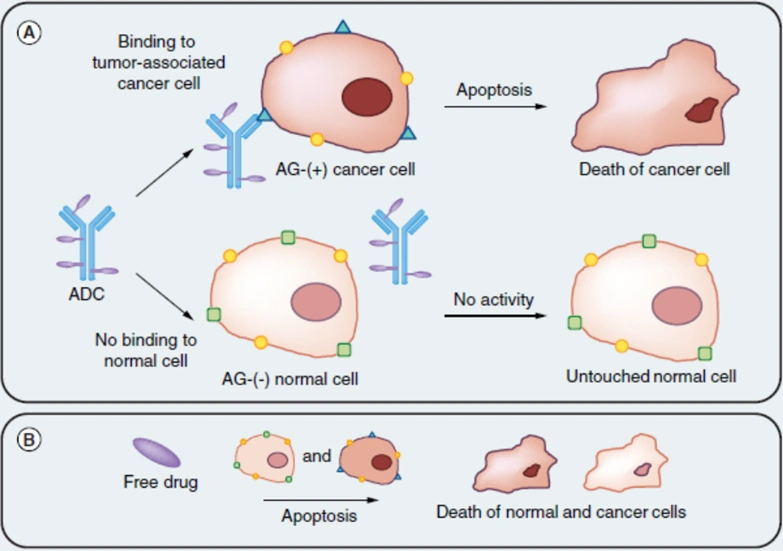
Leveraging the principles of both chemotherapy and immunotherapy, ADCs innovatively combine antibodies with chemotherapeutic drugs. Traditional chemotherapy is infamous for its off-target effects, often damaging healthy and cancer cells alike, due to the general mechanism of disrupting cell replication. Despite the faster replication rate of cancer cells, this does not spare healthy cells from chemotherapy toxicity. ADCs address this issue by coupling chemotherapeutic drugs with specific antibodies, designed to recognise receptors unique to or overexpressed in cancer cells. This precision allows ADCs to differentiate between cancer and non-cancer cells, embodying the “magic bullet” concept and minimising collateral damage to healthy tissues (Figure 1).
Figure 1. The general mechanism of an antibody-drug conjugate (ADC). (A) The ADC first binds to an antigen (AG) or receptor specific to cancer cells, releasing its conjugated cytotoxic agent into the cells and triggering apoptotic cell death. Normal cells without the cancer-specific antigen or receptor remain untargeted by the ADC. (B) In contrast, free cytotoxic drug often kills cancer and normal cells alike. Source: Creative Biolabs (2018).
In the last two decades, the Food and Drug Administration (FDA) has approved at least 15 ADCs to treat cancer. While the approval of the first few ADCs was intended for liquid cancers, namely leukaemia and lymphoma, more recent advancements have expanded the scope of ADCs to solid cancers, primarily of the breast, lung and stomach. Currently, over 100 ADC candidates are undergoing investigation in various clinical trials, showcasing their vast potential as the next frontier of cancer medicine.
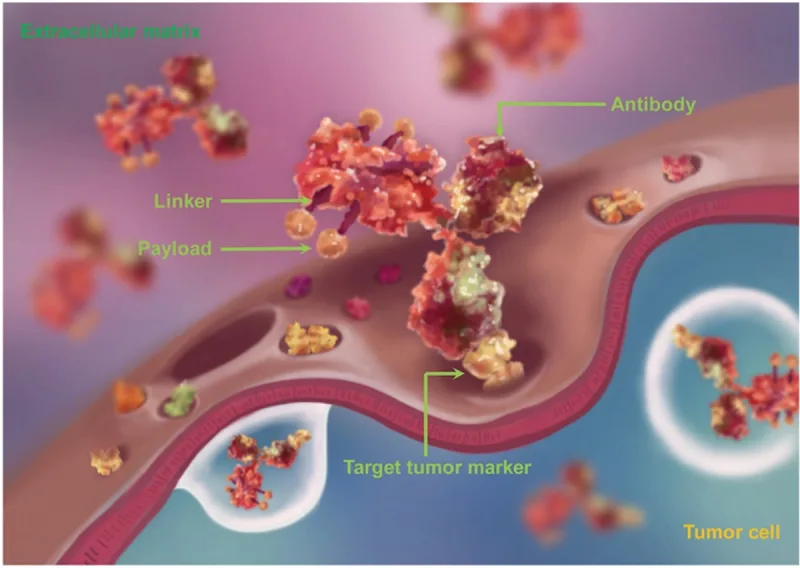
An example of ADC is gemtuzumab ozogamicin, approved for treating a specific type of liquid cancer, i.e., CD33-positive acute myeloid leukaemia (AML). This ADC consists of cytotoxic payloads (ozogamicin) linked to antibodies (gemtuzumab) targeting the CD33 receptor present on AML cells. The antibody-receptor binding facilitates the internalisation of the ADC into AML cells. In the cell, the ADC is transported to lysosomes, where its linker connecting the cytotoxic payload and antibody undergoes cleavage to release the cytotoxic ozagamicin. While ozogamicin is inherently toxic due to its ability to break apart cellular DNA, its conjugation with gemtuzumab (the CD33-targeting antibody) focuses its toxicity on AML cells. In essence, ADCs contain three fundamental elements: the tumour-targeting antibody, cytotoxic payload and linker (Figure 2).
Among solid cancers, breast cancer stood out as the main focus in the development of ADC, with at least four FDA-approved ADCs. These ADCs commonly target the human epidermal growth factor receptor 2 (HER2) specific to breast cancer cells. For example, trastuzumab emtansine and trastuzumab deruxtecan are ADCs that contain the trastuzumab antibody, which binds to HER2 to release its linked cytotoxic payloads, emtansine and deruxtecan, into the breast cancer cells. Emtansine disrupts cell replication while deruxtecan damages DNA, with both mechanisms ultimately triggering cell death. Hence, such an approach minimises harm to healthy cells without any expression of HER2 or other cancer-related receptors, for that matter.
Figure 2. The structure of an antibody-drug conjugate (ADC), consisting of an antibody, linker and payload (cytotoxin). The payloads are coupled or conjugated to the tumour-targeting antibody using linkers. Source: Jin et al. (2021), Pharmacology and Therapeutics.
The efficacy of these ADCs is not to be underestimated as well. In clinical trials, gemtuzumab ozogamicin prolonged event-free survival by eight months among CD33-positive AML patients compared to standard care alone, with 26% of the patients also achieving complete remission. Event-free survival denotes the duration a patient remains free from complications, such as disease progression, relapse or death; complete remission means the disappearance of all cancer signs. Similarly, trastuzumab emtansine successfully extended event-free survival by six months and increased complete remission rates by 13% compared to standard chemotherapy and immunotherapy in clinical trials of HER2-positive breast cancer patients. These results underscore the crucial role of ADCs in enhancing the effectiveness of current cancer medicine.
Challenges and future directions
One notable drawback of ADCs is the bystander effect, where the cytotoxic payload affects not only the targeted cells but also adjacent non-targeted cells. This phenomenon occurs when the cytotoxic payload diffuses out of the cancer cells and enters neighbouring cells, which may include healthy cells. As a result, drug toxicity has been observed in 5-20% of participants in clinical trials investigating ADCs, most commonly thrombocytopenia (low platelets) and neutropenia (low white blood cells). These complications can increase the risk of bleeding, infections or fatigue. However, such ADCs-related adverse events were still less frequent than those related to standard cancer therapies in clinical trials, indicating a more favourable safety profile.
Nevertheless, scientists have come up with solutions to minimise the bystander effect of ADCs:
- For one, the drug-to-antibody ratio (DAR) of ADCs can be reduced, which refers to the number of cytotoxic drug molecules attached to each antibody molecule. Reduced off-target toxicity has been observed with a lower DAR, which helps enhance tumour precision while limiting excess cytotoxic drugs from diffusing out of the cells.
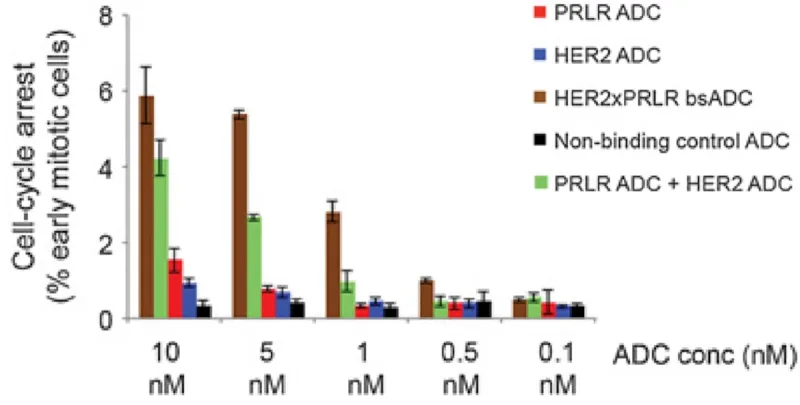
- Second, bispecific ADCs are being developed, which target two cancer-specific receptors to boost anti-cancer effects even at lower doses. For instance, ADCs targeting HER2 and CD63 or HER2 and prolactin receptors have shown improved specificity and efficacy at inhibiting breast cancer cells compared to ADCs targeting HER2 alone (Figure 3).
- Third, ADCs can incorporate cytotoxic drugs with limited ability to cross cell membranes. This design ensures that the drugs stay contained within the cancer cells, and should any leakage occur, their reduced capacity to penetrate cell membranes greatly diminishes the likelihood of affecting healthy cells.
Figure 3. Efficacy of various antibody-drug conjugates (ADCs) in inhibiting the replication of breast cancer cells by arresting the cell cycle. PRLR refers to prolactin receptor; HER2 refers to human epidermal growth factor receptor 2. Bispecific ADC (bsADC) targeting PRLR and HER2 (brown) exhibited the most potent anti-cancer effects, even at lower doses, compared to ADCs targeting PRLR (red) or HER2 (blue) alone and to co-administration of ADCs targeting PRLR and HER2 separately (green). Source: Andreev et al. (2017), Molecular Cancer Therapeutics.
Although ADCs still face other challenges involving their stability, biodistribution and tumour penetration capacity, these issues are not as urgent as the bystander effect. With the extensive research currently underway for ADCs, solutions to these obstacles may only be a matter of time.
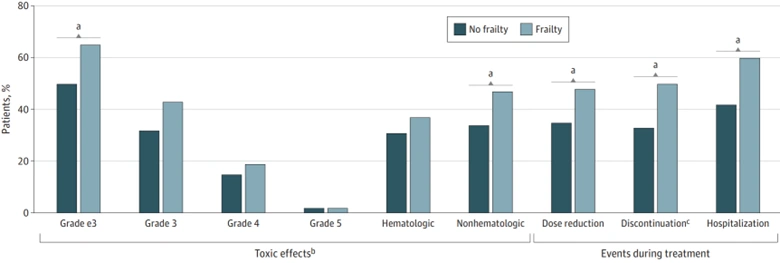
More pressing, however, are therapy-related off-target toxicities, which are a prevailing yet often underestimated problem in cancer care. These toxicities pose substantial psychological distress, financial burden and decline in life quality, which may also necessitate hospitalisations, dose reductions and even treatment termination (Figure 4). In this context, ADCs hold the promise of transforming cancer therapy by enhancing precision while reducing off-target toxicity. At last, Ehrlich’s vision of a “magic bullet” from over a century ago is finally materialising.
Figure 4. Incidence of various chemotherapy outcomes in cancer patients by frailty status. Source: Baltussen et al. (2023), Journal of the American Medical Association (JAMA) Network Open.