Problem Statement
Authorities such as the American Cancer Society, Centers for Disease Control and Prevention, and American Society of Clinical Oncology have advised that individual cancer patients seek medical consultation on the risks and benefits of receiving a vaccine for the coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Why is that when the vaccine is deemed safe for the general population?
An exclusion criterion of most, if not all, clinical trials investigating COVID-19 vaccines was participants with immunosuppression, which includes cancer patients who may be undergoing immunosuppressive therapies. This means that no clinical data have shown how COVID-19 vaccines would affect cancer patients. Would the vaccine still be as effective or, most importantly, safe as it should be? This article will draw on theoretical concepts and the current clinical evidence to clarify such concerns and, in light of the answers, the recommended precautions to keep cancer patients safe from both COVID-19 and the vaccine.
COVID-19 Vaccines: The Knowns and Unknowns
The Food and Drug Association (FDA) has approved two vaccines for emergency use for COVID-19, which are messenger RNA (mRNA) vaccines that Pfizer-BioNTech and Moderna developed. These two companies have achieved the fastest vaccine development in history, taking only about a year by capitalizing on the new mRNA technology. However, mRNA is not entirely new in the literal sense. Decades of research have already been put into understanding its basic biological properties to its complex functions and interactions with biosystems.
The mRNA vaccine consists of mRNA genetic material encapsulated in lipid nanoparticles. This mRNA codes for the spike protein of SARS-CoV-2 recognizable by the immune system. Upon arm injection, the lipid nanoparticles enter muscle and surrounding immune cells, releasing the mRNA into the cell. The cell’s protein manufacturing system will then read and translate the mRNA into spike proteins to train the immune system. Since it is only the spike protein and not the entire SARS-CoV-2 virion, no actual infection will happen. This immunization method works so well that the Pfizer-BioNTech and Moderna mRNA vaccines achieved a remarkable feat of 95% and 94.5% efficacy, respectively, at preventing COVID-19 in phase III clinical trials.
The World Health Organization (WHO) has also approved the AstraZeneca-Oxford vaccine with 76-82% efficacy, which uses the established method of adenoviral vaccine delivery previously used for tuberculosis, malaria, and Ebola virus. Other vaccines that gained approval for emergency use soon after that include the Sputnik V adenoviral, Johnson & Johnson adenoviral, Convidecia adenoviral, Sinophram inactivated, and Sinovac inactivated vaccines with the respective efficacy of 91.6%, 61-72%, 65.3%, 79.3%, and 50.7% at preventing COVID-19.
While the ~95% efficacious mRNA vaccine is joyous news, experts have raised concerns about the clinical trial protocol. One major concern is that only symptomatic COVID-19 was assessed. Only participants who developed symptoms were tested for SARS-CoV-2 to confirm a COVID-19 diagnosis. Therefore, the trials do not tell us whether the mRNA vaccine prevents infection, transmission, or disease progression into severe COVID-19. Not to mention that most vaccine trials excluded or recruited relatively fewer elderly participants, who are also the priority group at higher risk of severe COVID-19.
“You might think, ‘What does this matter? — if you can stop mild disease, then surely that will prevent severe disease as well,’ because first, you get infected and then you get symptoms and then it could get worse,” said Peter Doshi, an assistant professor specializing in drug regulation. “But while that’s true for a single individual, that’s not the case when you have many people, as in a trial. You can have a situation where you have an overall reduction in mild cases, but no reductions in hospitalizations, ICU [intensive care unit] use or deaths.”
However, judging from Israel’s success, which has fully vaccinated over half of its population, it seems that the mRNA vaccine also prevents severe COVID-19 in older adults. Of the vaccinated 750,000 adults over 60 years of age, only 531 got tested positive for SARS-CoV-2, of whom 38 were hospitalized for moderate-to-severe COVID-19, and three died. While this is an encouraging finding, the follow-up period is only one week following the second vaccine shot. Note that the mRNA vaccine follows a two-dose regimen taken 3-4 weeks apart. Nonetheless, at least this Israel data tells us that, once infected, there is a 7% (38/531 × 100 = 7%) chance of developing moderate-to-severe COVID-19 among vaccinated older adults. That is almost one in ten.
This is consistent with the recently published study in Israel detailing that the Pfizer-BioNTech mRNA vaccine reduced the risks of infection, symptomatic COVID-19, hospitalization, and severe disease by 92%, 94%, 87%, and 92% at one-week follow-up after the second vaccine shot. Such vaccine efficacy is indeed high, but it is also important to remember that the risk of hospitalization and severe disease is still about one in ten despite vaccination with the most effective vaccine we have.
While the intention is not to undermine vaccine success, there are still many unanswered questions requiring more attention:
- How effective is the vaccine at preventing SARS-CoV-2 transmission and protecting the most vulnerable populations (i.e., the elderly and persons with comorbidities) from severe COVID-19 in other countries besides Israel?
- How long does the vaccine-induced immunity last? In fact, companies, including Pfizer-BioNTech, are now developing another vaccine booster shot to maintain immunity against the increasing prevalence of diverse SARS-CoV-2 strains.
- Is the vaccine equally effective against the B.1.1.7 (British), B.1.351 (South African), P.1 (Brazilian), and CAL.20C (Californian) coronavirus strains that have evolved increased transmissibility and immune evasion capabilities? The AstraZeneca/Oxford vaccine, for example, is no longer effective against the South African strain.
- How is the vaccine’s safety profile in the long-term, especially for the new mRNA vaccine technology?
- How do children, adolescents, pregnant women, residents in nursing homes, or persons with immunosuppression or autoimmune disorders fare with the vaccine? Most, if not all, vaccine trials excluded these groups of people, so data on vaccine efficacy and safety for them is lacking.
As covering each of these would be overly extensive, this article will focus on the last point. The risk-benefit analyses of COVID-19 vaccines, particularly the mRNA vaccine, in cancer patients will be communicated.
Potential Risks of Vaccines in the General Population
Before focusing on cancer patients, it is imperative to note that the COVID-19 vaccines have a few risks applicable to the general population. After all, all drugs have risks that must be weighed against the risks of the disease.
A life-threatening allergic reaction called anaphylaxis occurring 15-30 minutes after receiving the mRNA vaccine can happen, especially to those with a history of allergies. But it is extremely rare; only 4.7 and 2.5 cases of anaphylaxis per million (0.00047% and 0.00025%) doses of Pfizer-BioNTech and Moderna mRNA vaccines, respectively, have happened. Fortunately, anaphylaxis is treatable with prompt epinephrine administration available on standby, so nobody died.
Among the 25 million persons who received the AstraZeneca/Oxford vaccine, 62 and 24 persons experienced blood clotting disorders in the brain (i.e., cerebral venous sinus thrombosis) and abdomen (i.e., splanchnic vein thrombosis), respectively, as of 22 March 2021. Of these 86 vaccine-induced blood clotting incidents, 18 resulted in death. Notably, such numbers are higher in certain countries, such as Denmark and Norway, at the rate of 11 excess blood clotting incidents per 100,000 vaccine doses. As a consequence, many European countries have restricted the use of the AstraZeneca/Oxford vaccine to the older age groups at greater risk for severe and fatal COVID-19. Vaccine-induced thrombotic thrombocytopenia (VITT) has also been acknowledged as a legitimate clinical disease.
Similarly, 20 cases of immune thrombocytopenia (i.e., a disorder where the immune system attacks the platelets that clot blood) have been reported among the 20 million persons vaccinated with the Pfizer-BioNTech mRNA vaccine in the U.S., of which one died. However, since reporting vaccine complications to surveillance systems is voluntary and much of the attention has been put on VITT, side effects and adverse events from other vaccines may have been overlooked. A 2006 systematic review of 37 studies found that as many as 90% of drug side effects were not reported unless investigations were deliberately conducted. For example, a 1995 study in The Lancet, a world-ranking medical journal, found that 82% of immune thrombocytopenia cases from the measles, mumps, and rubella vaccine were not reported to authorities.
However, more importantly, such rates of vaccine-induced blood vessel events pale in comparison to the actual threat of COVID-19. Of the total COVID-19 cases worldwide, about 2% have resulted in death, plus another 10-30% who became debilitated with the long-COVID syndrome. Not to mention that COVID-19 causes blood clots in 8% of patients, which goes up to 23% in those admitted to the ICU.
Theoretical concerns exist that the spike protein of SARS-CoV-2 may not be entirely harmless as we thought. The spike protein is also what current vaccines rely on to elicit immune responses for immunization against SARS-CoV-2. However, several studies have discovered that SARS-CoV-2 spike protein alone, without the whole virion or intact genome, is sufficient to cause blood vessel dysfunction in cultured cells and mice in laboratory settings. But whether such findings translate to humans or vaccine-related spike proteins remain unconfirmed.
“I am concerned about the possibility that the new [mRNA] vaccines aimed at creating immunity against the SARS-CoV-2 spike protein have the potential to cause microvascular [blood vessel] injury to the brain, heart, liver and kidneys in a way that does not currently appear to be assessed in safety trials of these potential drugs,” cautioned Patrick Whelan, MD, PhD, an assistant clinical professor and pediatric rheumatologist. This concern is further magnified considering that mRNA vaccines use lipid nanoparticles, which easily enter mammalian cell membranes. Plus, the tissue biodistribution of mRNA and DNA (AstraZeneca/Oxford) genetic vaccines – i.e., where the biomaterial ended up inside the body – remains understudied. This means that we are not quite sure where the genetic vaccines might go inside the body when injected.
But, speaking theoretically again, the spike protein expression only lasts for about 48 hours, at least for the mRNA vaccine, because mRNA is fragile and degrades very quickly. Since we already have about a year of vaccine safety data from the phase I clinical trial, the risk of mRNA vaccine causing blood vessel problems in humans is most likely not worrisome in general. However, as mentioned, most COVID-19 vaccine clinical trials excluded specific groups of people, such as immunocompromised persons. Thus, the potential risks of vaccine-related spike proteins causing blood vessel complications in specific individuals should not be disregarded entirely either.
Potential Risks of Vaccines in Cancer Patients
Persons with medical comorbidities, including cancers, are at increased risk of severe COVID-19. As the risks of not getting vaccinated are much greater, authorities advise that most cancer patients get vaccinated if there are no contraindications such as on-going immunosuppressive therapies or a history of vaccine-induced anaphylaxis (i.e., severe allergic reaction). The current authorized vaccines for COVID-19 do not use live or weakened viruses, so there would not be any risks of new infections upon vaccination in immunocompromised patients. However, authorities also did not dismiss that other vaccination risks may exist, recommending that cancer patients consult their doctors.
The vaccine’s main job is to stimulate and train the immune system. However, cancer is a highly heterogeneous condition that manifests and behaves differently in every case. Decoding the precise immune-cancer dynamics in an individual cancer patient is, therefore, difficult, especially when factoring in cancer progression and therapies. This indicates that each cancer patient may respond differently to vaccination.
For one, the immune system of cancer patients is actively battling the cancerous cells or tumour, preventing their enlargement and spread to other areas. The tumour wanting to survive, in turn, secretes various biochemicals to suppress the immune system. This renders cancer patients more susceptible to infections because of the limited available immunological resources. As follows, one possible risk is that vaccination may re-direct or recruit immune cells to the vaccination site to mount proper immunization responses. As a result, immune control of cancer cells or tumour growth elsewhere may weaken, providing an opportunity for cancer progression or recurrence.
This risk may be particularly concerning for older adults. Reduced peripheral T-cell and other immune activities have been documented following influenza vaccination in older persons and aged mice. T-cells are part of the adaptive immune system crucial for eliminating cancerous and virus-infected cells. Vaccination may, thus, use up the limited T-cell resources needed to control cancer growth in older adults. “Therefore, it is important to assure the vaccination would not cause a further T-cell exhaustion state which may have already been induced by tumour cells,” stated a 2021 research review in the journal International Immunopharmacology. However, there are ways to minimize such risks, as the subsequent section will elaborate.
Most cancer therapies are immunosuppressive. Chemotherapy and radiotherapy mainly kill cancer cells, but their toxicity often extends to neighbouring healthy cells. A common consequence of this is the depletion of immune cells. Surgical removal of the tumour also weakens the immune system due to the physical trauma that causes inflammation. Bone marrow or stem cell transplant therapy for certain cancers also leaves the immune system debilitated for months. Thus, experts have advised that cancer patients undergoing immunosuppressive therapies wait for a few weeks until the immune system stabilizes, such as having adequate white blood cell count, before getting vaccinated.
“Vaccines are important for patients with cancer, but they should not be given during periods of immunosuppression from chemotherapy and immunotherapy because, at such times, they may not be effective and live vaccines may result in vaccine-derived infections,” stated Patricia Hibberd, MD, PhD, a professor and chair of global health in 2021. Indeed, it is counterintuitive to expect effective immunization from vaccines when the immune system is still compromised.
A weakened immune system would also render vaccines less effective. This is confirmed in two separate 2021 studies finding that about half of the patients with liquid cancers (i.e., the blood, bone marrow, or lymph nodes) failed to deploy antibodies in response to the mRNA vaccine for COVID-19. Other studies published in recent months have also found that the immune system of patients with solid cancers (e.g., breast, pancreatic, skin, and prostate), especially those who are older and on chemotherapy, responded poorly to COVID-19 vaccination compared to persons without cancers. Only one of these studies examined vaccine safety in cancer patients, which, thankfully, found no signals of vaccine toxicity. But this is only one study with limited sample size and follow-up duration, so more safety data is always welcomed.
On that note, Ben Pfeifer, MD., PhD, professor and director of research at Aeskulap-International in Switzerland, has some clinical experience dealing with vaccinated cancer patients. He has preliminary data on 17 patients (6 females; 11 males; aged 42-76) with stage IV advanced cancer (i.e., most commonly breast, prostate, and colon) that were well-controlled for over five years following the Pfeifer protocol. Three to five weeks after getting the COVID-19 vaccine, however, these patients began to show signs of cancer progression, as tested via tumour blood biomarkers and imaging techniques. Prof. Pfeifer also reasoned that such setback might be due to adaptive immune cells being channelled to the vaccination site, which relaxes immune surveillance over the advanced cancer.
Interestingly, a case study published in Nature Medicine, another world-leading journal, described a 58-year-old male with stage IV colon cancer who got the Pfizer-BioNTech mRNA vaccine. Five days later, he manifested cytokine release syndrome (CRS), also known as cytokine storm (i.e., a severe systemic inflammatory disorder). Thankfully, his life was saved with anti-inflammatory drugs, and he now avoids the second vaccine shot. “The close temporal association of vaccination and clinical presentation favours the vaccine as the potential trigger of CRS in this case,” the authors concluded. Both this study and Prof. Pfeifer’s data show that vaccination has different risk profiles in different people, and patients with advanced cancers should be more careful.
Besides, any additional stimulation of the frail immune system via vaccination may pose other unknown risks. “Because some cancer patients are already weak and debilitated, there is a small chance that vaccine side effects could make them slightly more weak and debilitated, increasing their risks of other serious infections such as pneumonia,” agrees The Philadelphia Inquirer, a reliable and factual news outlet. The immune system-cancer dynamics are also incredibly complex that differ between individual cancer cases, and we do not know precisely how additional immune stimulation like vaccination would impact such dynamics in the short- and long-term.
All that said, how can we minimize such possible vaccination risks while maximizing vaccine efficacy in cancer patients?
Minimizing the Risks
To recap, currently, there are two main possible risks of vaccination in cancer patients:
1. Ineffective immunization with limited protection against COVID-19.
2. Unknown interactions between the vaccine and the delicate immune system, which may trigger unexpected adverse reactions.
Researchers have proposed various ways to enhance vaccine delivery and efficacy in cancer or immunocompromised patients, such as the usage of nanomaterial technology or vaccine inhalers. While such new vaccine technology is promising, they are still in the theoretical phase backed by experimental animal evidence only. Further clinical trials in humans are still required, which take time and resources. Such an approach also does not address the second possible risk mentioned above.
The current widely accepted recommendation for cancer patients is to get vaccinated when not on any on-going immunosuppressive therapies for reasons explained in the above section. Notably, this approach could also be complemented with immune-supporting nutrients and phytotherapy (i.e., plant-derived medicinal components).
Vitamin D, selenium, and zinc are some of the most well-known nutrients essential for maintaining a competent immune system. Although the specific immunological mechanisms vary, they all have roles in tuning down excessive inflammation while enhancing B-cell and T-cell responses of the adaptive immune system essential for controlling cancer and generating long-term immune memory against infections.
Unfortunately, nutritional deficiencies are prevalent worldwide today. Even when marginal, nutritional deficiencies could impair the immune system and lower vaccine efficacy. For instance, zinc deficiency is an independent factor of poorer vaccine responses against tetanus in children. Supplementing children with zinc has also been shown to improve the efficacy of the cholera vaccine. Similarly, vitamin D deficiency and supplementation have been linked to influenza and hepatitis B vaccine responses in adults. A similar pattern has also been seen with other studies involving selenium and vaccine responses.
Plant compounds have long been used as adjuvants to boost overall vaccine efficacy. This bears substantial significance because conventional vaccine adjuvants, such as aluminium salts and oil emulsions, can be toxic in susceptible persons. In contrast, plant compounds are natural in origin that humans have used for medicinal purposes since 2600 B.C in Mesopotamia without any major safety concerns. “Plant-derived immune-stimulatory compounds open the possibility to attain the main goal in adjuvant research: a safe and non-toxic adjuvant capable of strongly boosting and directing immune responses that could be incorporated into different vaccine formulations,” stated a 2019 research review. For example, Advax™, a plant inulin adjuvant, has shown success in enhancing the efficacy of influenza and hepatitis B vaccines in human clinical trials.
Therefore, the immunomodulatory properties of plant compounds can be capitalized on to improve overall health and complement conventional medicine, including cancer therapies and vaccines. Not only would plant compounds boost vaccine efficacy, achieving an adjuvant-like effect, but they may also minimize the potential risks of adverse vaccine interactions with the delicate immune system of cancer patients. For instance, the anti-inflammatory properties of plant compounds may dampen the excessive and unnecessary inflammation that vaccination sometimes induces. To this end, Prof. Pfeifer has recommended IMUSAN and BioBran MGN-3 Arabinoxylan.
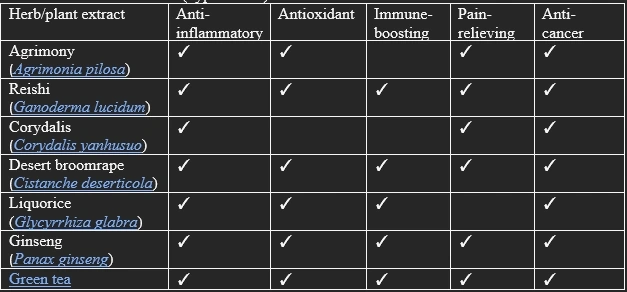
IMUSAN consists of 15 different herb extracts, which have been shown to possess various health-promoting biological properties (e.g., anti-inflammatory, antioxidant, immune-boosting, pain-relieving, and anti-cancer effects) in multiple studies involving mammalian cells or animals (Table 1).
Table 1. Published research (hyperlinked) on some of the herbs found in IMUSAN.
BioBran MGN-3, on the other hand, contains arabinoxylan as its main bioactive ingredient. Arabinoxylan is a type of short-chain polysaccharide from rice bran, widely researched by many since its patent in 1992. Mamdooh H Ghoneum, PhD, an associate professor and head of Immunology Research at Drew University, has commended BioBran as the most potent immunomodulator he has researched. The immunomodulatory and anti-cancer activities of BioBran have been demonstrated in mammalian cells, animals, and human clinical trials.
The benefits of IMUSAN and BioBran, as well as other supplementary products that the Pfeifer protocol recommends, in treating cancer are too expansive to detail here. So, this article will focus on their immunomodulatory activities relevant to vaccine responses instead.
As mentioned, the current COVID-19 vaccines all rely on the SARS-CoV-2 spike proteins to train the immune system. Dendritic cells (DCs) are very important in this process, as DCs are one of the early responders of the innate immune system that migrate to lymphoid tissues to alert the adaptive immune system of the spike proteins. This is because DCs are the most efficient type of antigen-presenting cells (APCs) that present the antigens (i.e., the spike protein, in this case) to the adaptive immune system. Adaptive B-cells and T-cells are then deployed to generate a memory of the spike protein, readying themselves against future encounters with the spike protein, such as during SARS-CoV-2 infection.
Interestingly, one of the ways in which BioBran exerts its anti-cancer effects is via DC stimulation. BioBran is capable of stimulating the cluster of differentiation 80 and 86 (CD80 and CD86) proteins present on DCs, resulting in the production of cytokines that activate B-cells and T-cells to kill or inhibit cancers. Giving BioBran to patients with multiple myeloma (i.e., a type of blood cancer) has been shown to increase levels of DCs upon blood analyses. Other studies have also detected increased B-cell and T-cell activities in various cancer patients (e.g., prostate, breast, colorectal, lung, and multiple myeloma) supplemented with BioBran.
“MGN-3 [BioBran] thus functions as a natural adjuvant for DC activation and may be used in DC-based vaccine strategies against infections and cancer,” stated a 2016 research review of Prof. Ghoneum. Recall that COVID-19 vaccines are also DC-based. Similarly, certain herbs found in IMUSAN, such as green tea, ginseng, and reishi, can also stimulate DC activities and the downstream immune responses that DCs activate.
Therefore, there is a basis for why BioBran and IMUSAN supplementation could help improve vaccine efficacy, on top of inhibiting cancer activities, in cancer patients who would also benefit greatly from additional immune support to their compromised immune system. Lastly, and perhaps the most important of all, BioBran and IMUSAN have been safely used in clinical practice to improve the health outcomes of cancer patients without any major adverse side effects.
Key Points
As the COVID-19 pandemic began in early 2020, inventing an effective vaccine has become a global priority. By employing the novel mRNA technology, COVID-19 vaccines with over 90% efficacy have been successfully developed in less than a year. However, precisely because it was so fast, many unanswered questions remain. For one, immunocompromised persons, including cancer patients, were excluded in most, if not all, the COVID-19 clinical trials, so vaccine safety and efficacy data on them is almost non-existent.
The immune system of cancer patients takes a massive toll from both the cancerous tumour and immunosuppressive therapies. As the vaccine’s main job is to train the immune system against a particular pathogen, a sub-par immune system would undermine vaccine efficacy. Moreover, stimulating the already debilitated immune system of cancer patients with vaccines may pose unknown risks; for instance, the vaccine may re-direct the limited immunological resources from controlling cancer to mounting vaccine responses.
Authorities, however, still recommend that cancer patients get vaccinated to protect against the more dangerous COVID-19. Therefore, it is imperative that strategies to minimize the potential risks of vaccines while maximizing vaccine efficacy be implemented for cancer patients.
One such approach is to ensure that any nutritional deficiencies are taken care of, especially vitamin D, selenium, and zinc that play critical roles in maintaining an optimal immune system. Phytotherapy is another powerful approach that capitalizes on the immunomodulatory and anti-cancer effects of selected plant compounds. BioBran and IMUSAN supplements are recommended for this purpose due to their capacity to stimulate DCs essential for swift activation of the adaptive immune system for effective vaccination and cancer control.
Overall, this article hopes to help cancer patients understand the potential risks they face when getting vaccinated against COVID-19. Although they are ‘potential’ risks that are only theoretical, it is never ill-advised to be cautious. Therefore, this article also arms the reader about what can be done to mitigate such risks. Hopefully, in the end, cancer patients who are getting vaccinated can be assured that they have done all they can to maximize vaccine efficacy against COVID-19 while minimizing its potential risks.