Night Shift Work and Cancer
In 2019, a team of 27 scientists from 16 nations gathered at the International Agency for Research on Cancer (IARC) in France to complete their assessment of the relationship between night shifts and cancer. They concluded that prolonged night shift work is “probably carcinogenic to humans,” grouped under 2A carcinogen, the same category as human papillomavirus infection, red meat consumption, and certain industrial chemicals. Similarly, in 2021, the U.S. National Toxicology Program (NTP) stated with “high confidence” that persistent night shift work, which disrupts the circadian rhythm, has carcinogenic effects on humans. This raises serious public health concerns, given that an estimated 18-20% of people work night shifts in America and Europe.
These conclusions were mainly derived from research on breast and prostate cancers. For instance, a pooled analysis of five independent studies from Australia, Canada, France, Germany, and Spain calculated that current or recent night shift workers had a 10-40% increased risk of breast cancer compared to those who never worked or ceased working night shifts for at least two years. The risk escalates to 2.5-fold if the night shifts are frequent (≥ 3 nights/week) and extend over a decade. Multiple studies have also reported a higher risk of prostate cancer among night shift workers. Predominantly, these workers are employed in the healthcare and service industries.
While the exact cancer risks linked to night shift work vary based on situations, both the NTP and IARC reports suggest that individuals who frequently work at night are at risk of cancer:
- Working night shifts that include at least three hours between midnight and 5:00 AM.
- Regular night shifts, occurring three or more times a week.
- Prolonged duration of night shift work, spanning 10 years or more.
- Starting night shift work at an early age, notably before turning 30.
Your Circadian Rhythm Matters
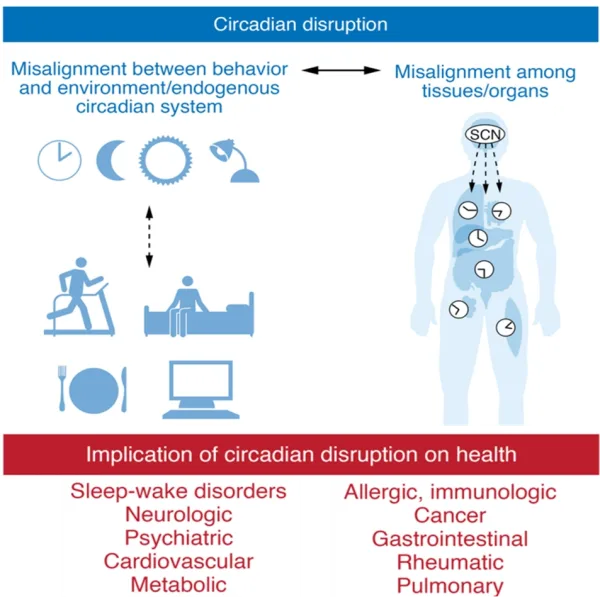
While night shift workers tend to have additional cancer risk factors (e.g., work stress, unhealthy lifestyle, and lower vitamin D levels due to reduced sunlight exposure), the main reason predisposing them to cancer is the disruption of the circadian rhythm. This internal clock is located in the suprachiasmatic nucleus in the brain, which regulates various physiological processes (e.g., hormonal cycles, immune responses and cell growth) on a 24-hour daily basis. However, external signals, such as light, temperature and dietary intake, can affect the circadian rhythm for the better or worse. Misaligned circadian rhythm has been linked to various diseases, including cancer (Figure 1). Importantly, circadian disruption affects not only shift workers but also those who regularly experience poor sleep by waking for several hours during the night, often due to jet lag, excessive intake of alcohol or caffeine, or exposure to blue light from screens.
Figure 1. A schematic depiction of the relationship between circadian rhythm disruption and human diseases. Note: SCN refers to the suprachiasmatic nucleus, the brain area that controls circadian rhythm. Source: Fishbein et al. (2021); The Journal of Clinical Investigation.
At the cellular level, the circadian rhythm influences various cellular processes, notably the cell cycle timing. The cell cycle refers to a series of events that a cell undergoes to grow and divide into two identical cells. Disruption of the circadian rhythm can cause irregularities in the cell cycle, which may lead to uncontrolled cell growth and the formation of cancerous mutations. As Dr Satchidananda Panda, PhD, a professor and circadian biologist at the U.S. Salk Institute for Biological Studies, explained, “When the clock gets disrupted, cells don’t know when to divide; they can divide faster and become a tumour.”
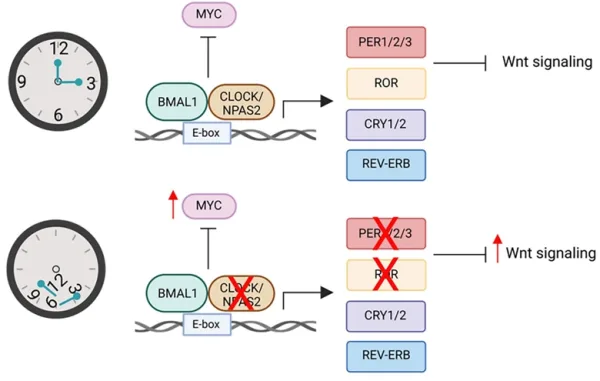
At the molecular level, extensive genetic analyses have found that circadian genes are significantly altered across several tumour types. The circadian rhythm is governed by activator and repressor genes. During the day, activator genes kick-start the circadian cycle and relevant physiological activities; at night, repressor genes dampen these activities. Disrupting this intricate balance can lead to the overactivation of certain pro-cancer genes, such as c-MYC, that drive cell proliferation. In turn, overactivated c-MYC can disturb the equilibrium between the circadian activator and repressor genes, perpetuating a vicious cycle and creating a pro-tumorigenic environment (Figure 2). The circadian clock also regulates gene expression related to immunological and hormonal activities, which also contribute to the development and aggressiveness of cancer.
Given the extensive influence of the circadian rhythm on our physiological functions, maintaining a synchronised circadian clock is crucial. Regular night shift workers, in particular, need to be more vigilant and adopt healthier habits (e.g., eating well, exercising regularly, and avoiding smoking and heavy drinking) to counteract the negative effects of night shifts on cancer risk. Warning signs of a disrupted circadian rhythm may manifest as severe fatigue or drowsiness during wakefulness, sleep problems, gastrointestinal issues, mood swings and impaired alertness. While prevention is best, realigning a disrupted circadian clock can be achieved through strategies such as increasing sunlight exposure and adhering to a regular schedule for sleep, meals and exercise.
Figure 2. The interplay between the circadian clock and cancer genes. Top: Under proper balance of circadian genes BMAL1 and CLOCK, the common pro-cancer gene MYC is inhibited (denoted by the ––| symbol). Bottom: A disruption of circadian genes leads to increased activity of the pro-cancer MYC gene, triggering downstream reactions that amplify the Wnt signalling pathway. This pathway promotes the growth of cells and blood vessels for cancer development. Source: Kaakour et al. (2023), Clinical Medicine Insights: Oncology.
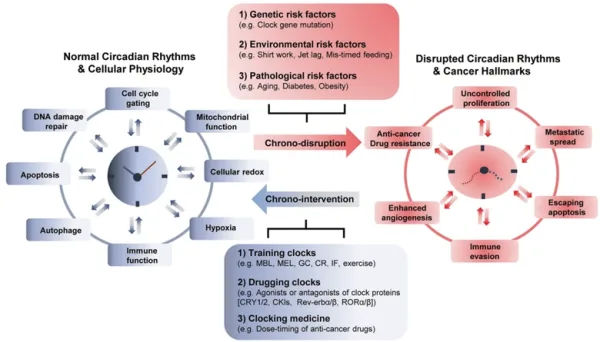
Given the intricate relationship between the circadian rhythm and cancer on the cellular and molecular levels, scientists are beginning to leverage this knowledge in cancer therapy. Currently, chronotherapy (chrono- means time) involves three approaches (Figure 3):
- Training the clock: Strategies to strengthen or preserve a consistent circadian rhythm, focusing on regular patterns of eating-fasting, sleep-wake cycles, and light-dark exposure.
- Clocking the drugs: Timing the administration of medications according to the circadian clock to enhance effectiveness and minimise negative side effects.
- Drugging the clock: Using small-molecule compounds that specifically target the processes of the circadian clock.
By training the clock, studies have demonstrated that increasing daylight exposure could enhance the nighttime release of melatonin and reduce tumour growth in animal models of liver, breast and prostate cancers. Additionally, a cohort study observed that daytime exercisers had a lower risk of developing prostate and breast cancers compared to nighttime exercisers or inactive individuals. Further research is underway to determine whether manipulating the timing of daylight exposure, exercise and feeding patterns can prevent or delay the growth of cancer.
Clocking the drug refers to manipulating the timing of therapy. Several clinical studies have found significant differences in the toxicity of chemotherapy and radiotherapy based on when it was administered. Depending on the therapy, morning intervention generally produces fewer adverse effects and more favourable clinical outcomes. On the other hand, drugging the clock involves using synthetic drugs to target specific genes and proteins of the circadian rhythm to mediate anti-cancer effects. For instance, casein kinases 1 and 2 (CK1 and CK2) are proteins that regulate the circadian cycle; however, suppressing these proteins with small-molecule inhibitors has been found to inhibit the growth of various cancers in pre-clinical settings.
Figure 3. Factors disrupting the circadian rhythm and chronotherapeutic approaches in cancer. The circadian clock interacts with various pathways critical for cell metabolism. Top: Disruptions in the circadian rhythm due to genetic, environmental and disease-related factors can lead to cancer development and progression. Bottom: Several chronotherapeutic strategies can restore the circadian rhythm to mitigate cancer development and enhance the efficacy of cancer therapies. Note: MBL, morning bright light; MEL, melatonin; GCs, glucocorticoids; CR, caloric restriction; IF, intermittent fasting. Source: Lee et al. (2021), Experimental and Molecular Medicine.
Aside from pharmaceutical interventions to ‘drug the clock,’ a more natural alternative to achieve this is through phytotherapy, the use of plant compounds for therapeutic purposes. The Pfeifer Protocol, for example, comprises a curated selection of plant compounds known for their bioactive properties, including the capacity to modulate the circadian clock:
- Lycopene (present in tomatoes): Two separate studies in 2022 and 2018 demonstrated that feeding mice with a diet containing lycopene or tomatoes significantly decreased tumour growth and gene expression involved in carcinogenesis, including several circadian genes.
- Quercetin (present in fruits and vegetables): A 2021 study disrupted the circadian rhythm of mice with irregular light-dark conditions, which accelerated the growth and spread of breast cancer. However, a diet containing quercetin significantly inhibited the cancer spread to other organs compared to the control group.
- Epigallocatechin-3-gallate (EGCG; found in green tea): A 2020 study observed that the gene expression of CLOCK, a crucial circadian gene, was dysregulated in early lung cancer cells. Silencing this gene or administering ECGC, however, managed to prevent the cells from turning cancerous.
In summary, the critical role of the circadian rhythm in cancer development and treatment highlights the need for increased awareness and proactive measures in managing our internal clocks. This is particularly crucial for individuals such as night shift workers, who are more susceptible to circadian disruptions. By aligning with natural light-dark cycles, maintaining consistent feeding, exercise and sleep schedules, and, when necessary, using phytotherapeutic supplements, we can significantly lower the risk of cancer.