Ageing may not simply be what happens when your body wears out. Emerging research suggests that the brain may also make strategic decisions about what to give up first: where to spend energy and where to cut back. Muscles shrink, hormones drop and even motivation fades — not just from damage but because the brain starts conserving fuel by shutting down systems that seem less essential. In this view, ageing is a deliberate shift in priorities orchestrated by the brain. This newsletter explores the compelling idea behind that theory, called the brain-body energy conservation model, and highlights promising strategies to rebalance the body’s energy equation and promote healthier ageing.
The Energy Paradox in Ageing
Most people assume that as we age, our cells gradually slow down and use less energy. However, new research reveals the opposite: senescent cells that accumulate with age actually increase their energy use and become hypermetabolic. In other words, they burn more fuel than they did when they were young. This may seem paradoxical since senescent cells have stopped dividing, mimicking a state of cellular retirement. Yet, beneath the surface, these cells become highly active in secreting inflammatory and stress signals. As a result, senescent cells have also been referred to as zombie cells: dysfunctional, yet unable to die.
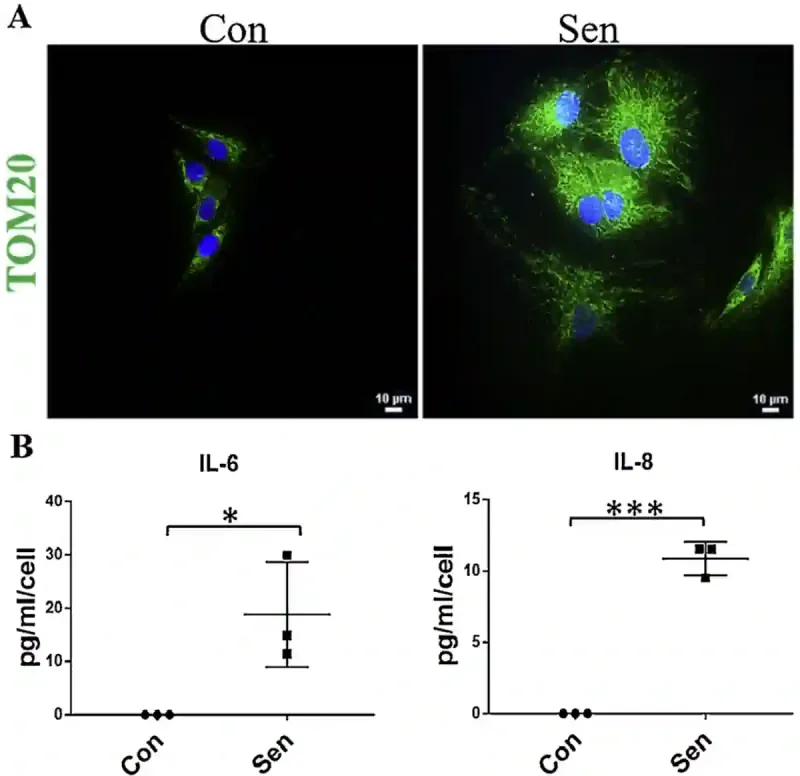
Research has found that skin cells from elderly individuals contain almost 50% more mitochondrial mass and protein content compared to those from young adults. Mitochondria are the cell’s energy factories, so an increase in their number reflects an elevated energy demand. Further laboratory experiments showed that when cells are deliberately aged through wear-and-tear damage, they respond by increasing inflammation and making more mitochondria. For example, one study exposed human cells to hydrogen peroxide (a toxic chemical used to induce oxidative stress) to accelerate cell ageing. Consequently, the damaged cells became senescent and began secreting inflammatory cytokines (interleukins) and producing more mitochondrial units compared to non-aged cells (Figure 1).
But why are aged cells using up more energy? Where is it all going? In a landmark 2024 paper published in Nature Ageing, Shaulson et al. from Columbia University’s Irving Medical Centre, New York, have proposed a few explanations based on current evidence.
- Compensating for inefficient mitochondria: As cells age and become senescent, their mitochondria become less efficient. To make up for this, cells generate more energy just to meet basic needs. This inefficiency means they burn more fuel to achieve less. A 2023 study found that cells with damaged mitochondria used up to twice as much energy per cell division, and they still divided more slowly.
- Powering the hypersecretory state: Senescent cells do not just sit still. They actively pump out stress signals, inflammatory molecules and other proteins in large quantities. This hyperactivated secretion mode demands vast amounts of energy for producing, processing and exporting these signals, which must be continuously replenished.
- Keeping up with repair and maintenance: Even though senescent cells no longer divide, they work overtime to clean up internal damage, recycle broken components and maintain cellular stability. This process involves breaking down and rebuilding proteins, lipids and other molecules. Like an ageing building that requires constant repairs, these internal renovations consume substantial energy every day.
Figure 1. Senescent cells have more mitochondria and release more inflammatory signals. (A) Senescence cells (labelled ‘Sen’) contain more mitochondria than healthy control cells (‘Con’), as shown by the brighter green signal (i.e., TOM20 biomarker) in the fluorescence microscopy images. (B) These senescent cells also release more inflammatory molecules (i.e., IL-6 and IL-8) compared to healthy cells, suggesting they are in a stressed or inflamed state. Abbreviation: IL, interleukin; TOM20, translocase of the outer mitochondrial membrane 20. Source: Patil et al. (2019), Mechanisms of Ageing and Development.
Here is the energy paradox of ageing: older cells need more fuel just when the body has less to spare. This creates a silent but serious energy shortfall. So, what happens when the costs of maintaining senescent cells start to outweigh the body’s energy budget? That is when the brain steps in. It starts to reallocate energy away from less essential systems to protect the more vital ones. In other words, the brain decides what it can let go of, shaping how and where we age.
The Brain-Body Energy Conservation Response in Ageing
The brain may be viewed as the body’s chief financial officer, constantly balancing the books of energy income and expenses. When damaged and aged cells start demanding extra fuel, they release cytokines (inflammatory messengers) and metabolites into the bloodstream. These signals travel to specialised brain regions in the hypothalamus and brainstem, which are responsible for sensing and managing energy balance in the body.
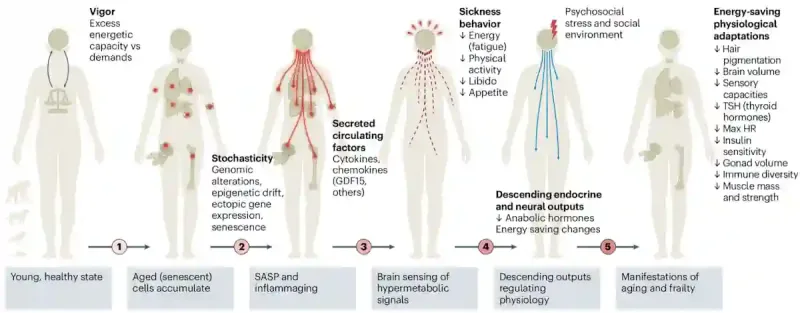
Once the brain detects a spike in these energy demand cues, it initiates what Shaulson et al. call the “brain-body energy conservation response” (Figure 2). The brain acknowledges the increased energy demand, implements cutbacks on less essential systems (e.g., muscles and hormones), and reallocates energy expenditure to the most vital organs (e.g., basic metabolism and heartbeat). This energy conservation response by the brain is not a malfunction. It is doing exactly what it has evolved to do – keeping the host alive with a limited energy budget. The result is a predictable sequence of cutbacks that shapes the trajectory of ageing:
- Muscle Atrophy: Muscle tissue is one of the body’s biggest energy consumers. By reducing muscle mass, the brain saves on the energy cost of upkeep and movement.
- Endocrine Decline: Hormones like thyroid hormone, testosterone, oestrogen, insulin and growth hormone are important for growth, repair and efficient metabolic activities. Downregulating these hormonal signals conserves fuel but also leads to thinning bones, slower metabolism and dwindling libido.
- Loss of Motivation: Behaviours like physical activity, socialising and libido require some energy investment. Dampening drive and reward circuits for these behaviours help minimise energy expenditure on non-vital pursuits.
- Immune System Simplification: A robust, adaptive immune response is energy-intensive. The brain scales back certain immune functions, especially costly antibody production, to preserve resources, even though this can increase the risk of infection.
- Brain Shrinkage: Neurons and supporting glia are metabolically expensive. In advanced age, the brain may downregulate neuronal growth factors and cognitive efficiency for lower energy demands.
- Others: Hair greying, sensory decline (e.g., hearing and vision) and fatigue all reflect the brain tuning down non-essential functions to conserve energy.
Beyond these usual signs of ageing, the brain’s energy conservation also comes with increased cancer risk. As more aged cells build up, they release a cocktail of inflammatory signals and growth factors, which can encourage cancer formation in neighbouring tissues. At the same time, the brain’s energy-saving mode dampens the immune system, including T-cells and natural killer cells vital for cancer surveillance. The result is a double hit: cells become more prone to cancer formation, while the body’s anticancer defences grow weaker.
Figure 2: The brain-body energy conservation model. As we get older, our cells build up damage and become less efficient. To cope, they release stress and inflammatory signals. The brain senses these signals and interprets them as a need to conserve energy. In response, it reduces energy-intensive functions, such as physical activity, reproductive drive and even appetite, in a process similar to sickness behaviour. Over time, this leads to broader changes in hormones, metabolism and body function. These energy-saving responses may help survival but also contribute to common signs of ageing, such as fatigue, weaker muscles, lower hormone levels and slower cognition. Source: Shaulson et al. (2024), Nature Ageing.
Rebalancing the Energy Equation
If ageing or senescent cells are consuming more energy than available, targeting these cells would rebalance the energy equation of the body and minimise energy cutbacks from other systems, including immune defences against cancer. As a result, scientists have explored ways to eliminate or inhibit these senescent cells, and three strategies have shown promise thus far: senolytic therapies, calorie restriction and exercise.
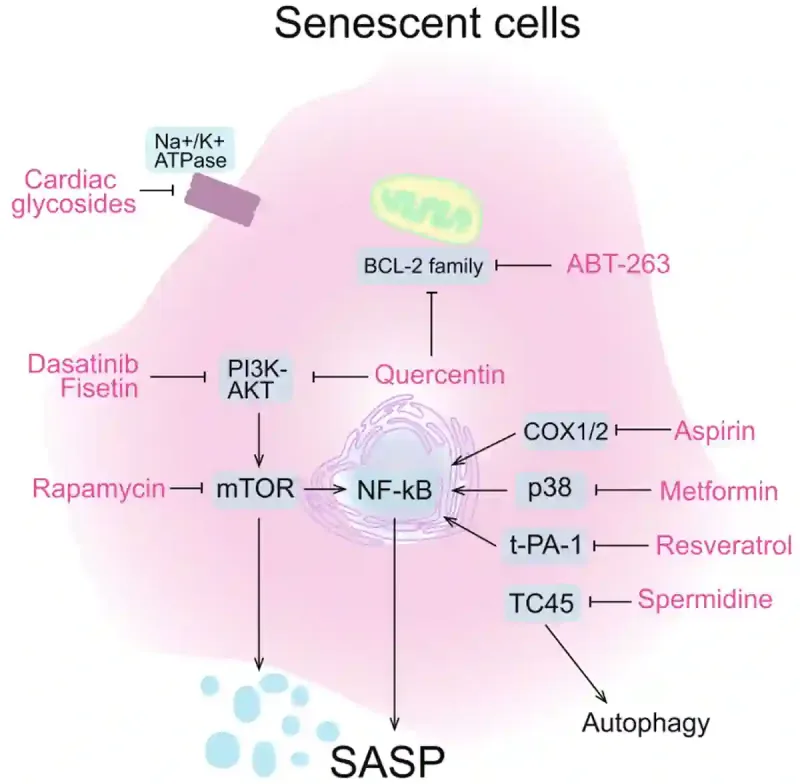
Senolytic therapies are compounds that remove senescent cells. One of the earliest and most studied senolytic therapies is dasatinib plus quercetin. Dasatinib is a chemotherapeutic drug used to treat leukaemia, while quercetin is a natural plant compound found in sources like apples, onions and berries. This duo works together to kill senescent cells by targeting different survival pathways these cells use to avoid death (Figure 3). In animal models, this combination has shown strong efficacy in clearing senescent cells and rejuvenating tissue function. It is now being tested in human clinical trials for age-related diseases such as dementia, pulmonary fibrosis and osteoporosis. While other synthetic (e.g., metformin and rapamycin) and natural (e.g., fisetin and resveratrol) compounds have also shown senolytic activity (Figure 3), dasatinib plus quercetin remains the most clinically advanced senolytic therapy to date.
Next, calorie restriction (without malnutrition) is one of the most effective and well-studied ways to delay ageing and extend lifespan. It primarily works by inducing autophagy, the body’s built-in recycling system. When nutrients are scarce, the body starts breaking down old or damaged cell parts and reuses them for energy. This clean-up process helps clear out faulty components before they accumulate and become senescent cells, which drain energy and fuel inflammation. Interestingly, some compounds can mimic the effects of calorie restriction by acting on the same cellular pathways. These include rapamycin (an immunosuppressant), metformin (an anti-diabetic drug) and resveratrol (a plant compound found in grapes and berries). They work by targeting the same nutrient-sensing pathways as calorie restriction, such as mTOR and sirtuins, thus promoting autophagy and cellular longevity.
Moving on, not all energy demands affect ageing in the same way. While chronic stress and inflammation speed it up, moderate exercise does the opposite, even though it also increases energy use. According to Shaulson et al., the difference lies in how energy is spent: exercise raises active energy use (like moving and lifting), not resting energy use (like maintaining organ function and senescent cells). This kind of demand triggers the body to adapt by building more mitochondria in healthy cells and shifting resources toward systems that support long-term health. These changes help improve organ efficiency and stress response while also preserving muscle and brain function with age. In other words, exercise tells your brain that the body is thriving and to invest in repair, not energy cutbacks.
Figure 3. How various senolytic agents inhibit the cellular pathways of old senescent cells. The agents include synthetic drugs (e.g., ABT-263, aspirin, rapamycin, metformin and dasatinib) and natural plant compounds (spermidine, resveratrol, fisetin and quercetin). By blocking certain cellular pathways, these senolytic agents either trigger senescent cells to self-destruct or dial down their inflammatory signals, restoring energy balance and freeing energy for healthier cells. Note: SASP stands for senescence-associated secretory phenotype, which refers to the hyperactive state of senescence cells in secreting inflammatory and stress signals. Source: Yu et al. (2024), Cell Regeneration.
Conclusion
The brain-body energy conservation model offers a new perspective on ageing: not as passive decline, but as the brain managing limited energy by making trade-offs. This shift opens the door to practical ways we might age better. Clearing senescent cells, triggering autophagy through calorie restriction, and exercising in ways that signal vitality can help rebalance energy use. Moreover, these steps may also lower cancer risk by reducing inflammation and restoring the immune system’s ability to detect and eliminate cancer cells. It is also encouraging that certain plant compounds can help clear senescent cells and activate the same autophagic pathways triggered by calorie restriction. This reinforces the growing value of phytotherapy, the use of plant-based compounds for therapeutic benefits. Ultimately, ageing may be inevitable, but how we age could be more within our control than we thought.