Revolutionary Anti-Cancer Vaccines: A New Frontier in Cancer Therapy
Background: The Past
Traditionally, vaccines are used to prevent infectious diseases by exposing the immune system to a specific component of the pathogen beforehand. As a result, vaccines are often associated with prevention in our lexicon. However, scientists are beginning to challenge old views by exploring the concept of therapeutic vaccines to treat existing diseases, such as cancer.
Interestingly, the first-ever use of therapeutic vaccines was intended to treat cancer, although it was not called a vaccine at that time. In a 1985 clinical trial, American scientists isolated colon tumour cells from cancer patients who underwent colon resection surgery. These tumour cells were re-infused into the patients after surgery. At 28 months of follow-up, only 15% of patients receiving this therapy had cancer recurrence and 0% died. In comparison, as many as 45% of patients in the control group had cancer recurrence and 20% died.
The development of anti-cancer vaccines took another pivotal turn when Belgian scientists identified melanoma-associated antigen 1, a protein specific to skin cancer that is recognisable by the immune system. This discovery was paramount as it means vaccines can be designed based on specific tumour antigens or proteins to immunise patients, rather than relying on whole tumour cells that pose the risk of causing secondary cancers.
Anti-Cancer Vaccines: The Present
After decades of progress, several anti-cancer vaccines have been successfully developed and approved by the Food and Drug Administration (FDA). Currently approved anti-cancer vaccines can be categorised into two main types: cell-based and virus-based.
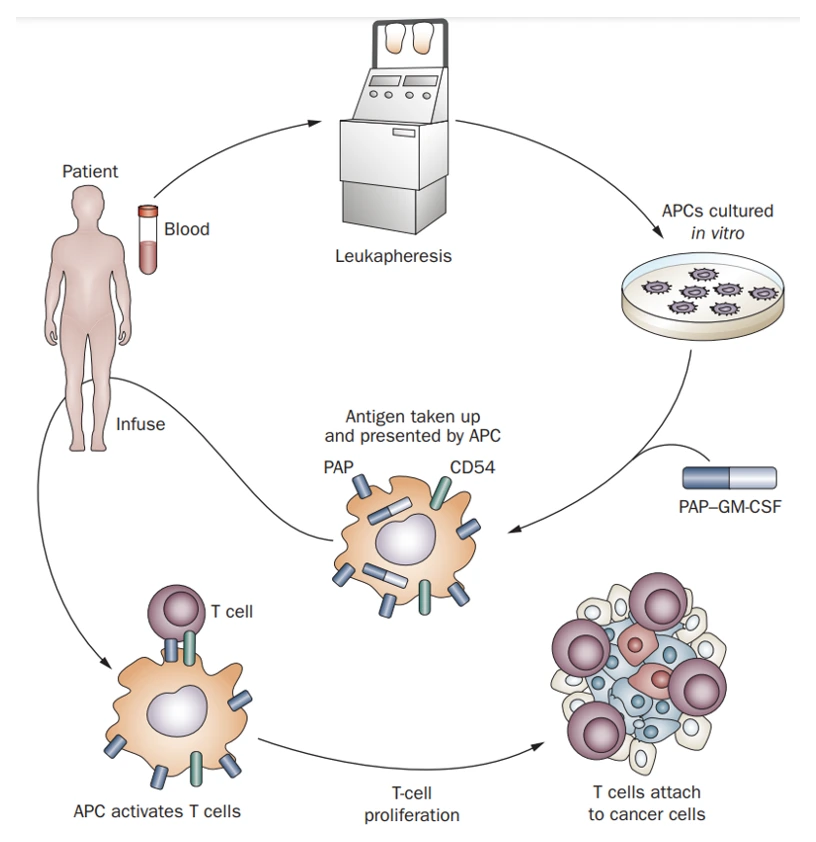
Sipuleucel-T was the first cell-based vaccine that the FDA approved for treating prostate cancer in 2010. In a major clinical trial, Sipuleucel-T reduced the risk of death by 22% and prolonged survival by four months compared to placebo among men with metastatic prostate cancer. This vaccine is created by first isolating dendritic cells (DCs) from the patient. DCs are responsible for presenting foreign antigens to T-cells, thus activating T-cells to destroy the foreign entity, such as the tumour. The isolated DCs were exposed to an antigen found in prostate cancer cells, namely prostate acid phosphatase. DCs carrying these antigens are re-infused into the patient, which then activate T-cells to kill prostate cancer cells expressing the antigen (Figure 1).
Figure 1. The general mechanism of Sipuleucel-T, a dendritic cell-based anti-cancer vaccine. Key abbreviations: APCs, antigen-presenting cells, which are mostly dendritic cells; PAP, prostate acid phosphatase, i.e., the antigen found in prostate cancer cells. Source: Di Lorenzo et al. (2011), Nature Reviews Clinical Oncology.
Although Sipuleucel-T is the only FDA-approved cell-based vaccine against cancer, numerous clinical trials are underway to examine other cell-based vaccines against pancreatic-, breast-, brain- and other cancers. Cell-based vaccines, however, are limited by their high costs and complicated processes of isolating, culturing and training dendritic cells. This brings us to another promising and more cost-effective anti-cancer vaccine platform: virus-based vaccines.
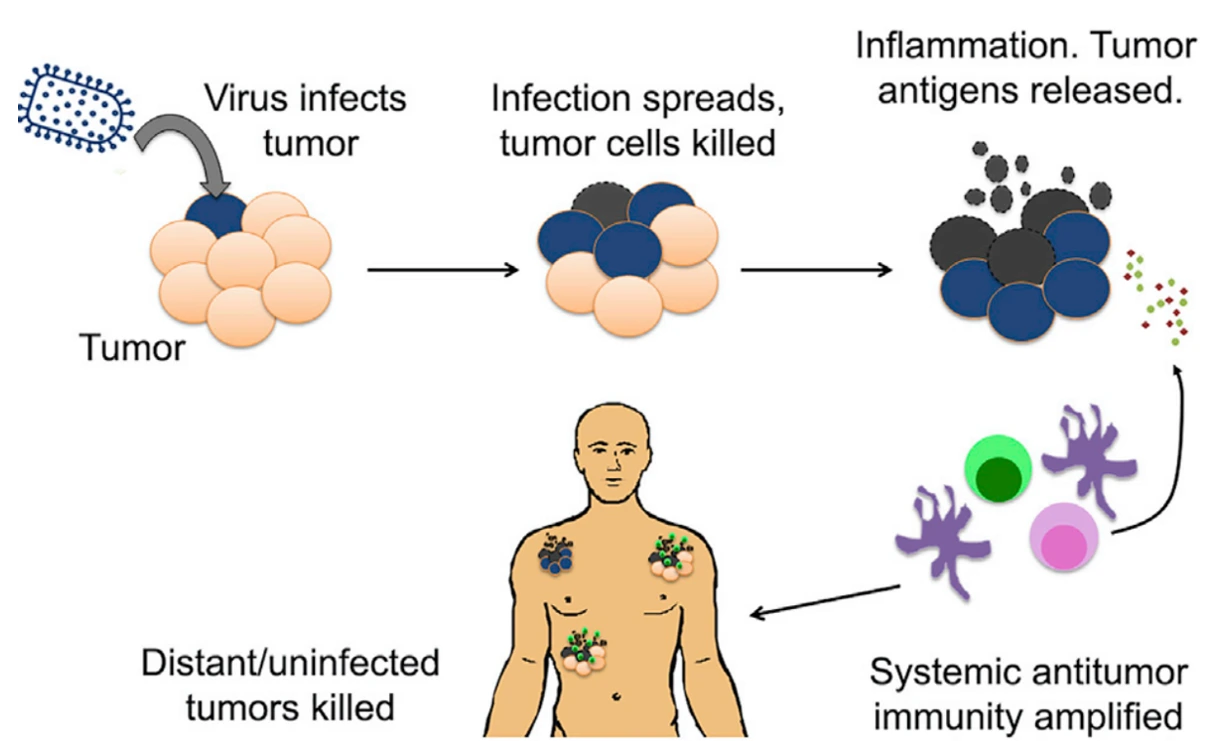
At present, the FDA has approved one virus-based vaccine, i.e., talimogene laherparepvec (T-VEC), for treating skin cancer in 2015. In a landmark clinical trial, T-VEC prevented cancer progression in 16% of patients with metastatic skin cancer; in comparison, the rate of cancer progression in the control group is at a staggering 98%. T-VEC is an attenuated herpesvirus that is genetically modified to infect and replicate selectively in tumour cells. This process eventually kills and lyses the tumour cells, releasing tumour antigens that further stimulate DCs and T-cells (Figure 2). The herpesvirus in T-VEC is also engineered to produce granulocyte-macrophage colony-stimulating factor (GM-CSF), a protein that enhances immune responses directed against the tumour. Such an approach is also called oncolytic virus therapies.
Aside from oncolytic viruses, virus-based vaccines can also use genetically engineered viruses that encode tumour antigens. Instead of infecting and killing tumour cells, these vaccines infect and induce dendritic cells to express the encoded proteins. This bypasses the arduous steps of isolating and culturing dendritic cells from the patient, as required for cell-based vaccines. The virus-infected dendritic cells then present the tumour antigens to T-cells, thereby activating a cytotoxic immune response against the targeted tumour.
Figure 2. The general mechanism of talimogene laherparepvec (T-VEC), an oncolytic virus-based vaccine. Source: Russel and Peng (2017), Molecular Therapy.
One unique benefit of anti-cancer vaccines is the formation of long-term immunity. Upon activation by anti-cancer vaccines, some T-cells differentiate into memory T-cells, which ‘remember’ the tumour and form anti-tumour immunity. This is a basic vaccinology principle, where vaccines are designed to train the immune system to prepare for a potential future encounter with a particular foreign threat. As a result, patients receiving anti-cancer vaccines have benefited from reduced risk of cancer recurrence in clinical trials.
Untapped Potential: The Future
Another fundamental problem that anti-cancer vaccines manage to solve is tumour specificity. Traditional cancer therapies, such as chemotherapy and radiotherapy, suffer from non-specificity and often harm healthy cells alongside cancer cells. This problem is also known as off-target effects. As a result, such cancer therapy modalities are notorious for their toxic side effects, such as vomiting, cognitive impairments and fertility problems.
Anti-cancer vaccines, however, circumvent this non-specificity issue by honing in on tumour antigens, which are unique to the tumour involved. This approach minimises collateral damage to healthy cells. For example, in the Sipuleucel-T and T-VEC clinical trials, the side effects documented were usually mild flu-like symptoms without any fatal side effects.
Due to their exceptional specificity, anti-cancer vaccines could reshape personalised cancer therapy. Scientists are currently developing anti-cancer vaccines using resected tumour tissues from patients. This method allows the identification of tumour antigens unique to each patient, which are then used to design personalised anti-cancer vaccines.
If successful, this strategy could be a breakthrough in overcoming tumour heterogeneity. Even among patients with the same cancer, their tumours often display distinct behaviours and mutation profiles. This diversity is one reason treatment results usually vary from one patient to another. Therefore, by targeting patient-specific tumour antigens, personalised anti-cancer vaccines offer an ingenious approach to stimulate the patient’s immune system to fight their specific cancer.
In conclusion, anti-cancer vaccines mark a profound shift in cancer therapy, harnessing vaccinology’s principles to deliver more effective, personalised and less harmful therapies. As research continues to advance, the potential of anti-cancer vaccines to revolutionise cancer therapy is becoming a reality, representing a significant milestone in medical science.