In our prior newsletter, we covered the basics of benign prostatic hyperplasia (BPH) – its symptoms, risk factors and current treatment strategies. While standard treatments can provide symptom relief, they often overlook the long-term outcomes and well-being of BPH patients. In this second issue, we will focus on plant extracts that take a more holistic approach to prostate health. Could plant extracts like saw palmetto help ease urinary symptoms of BPH while minimising side effects and improving overall health?
Lifestyle risk factors exist for BPH, a condition where the prostate gland enlarges and blocks urine flow. As outlined in our first newsletter, metabolic syndrome and poor diet are significant contributors to BPH risk. Metabolic syndrome refers to a cluster of interrelated conditions – including obesity, insulin resistance, hypertension and/or abnormal triglyceride and cholesterol levels – often driven by unhealthy lifestyle factors like a low-quality diet. These metabolic disruptions facilitate systemic inflammation and hormonal imbalances, both of which create an environment that promotes prostate enlargement.
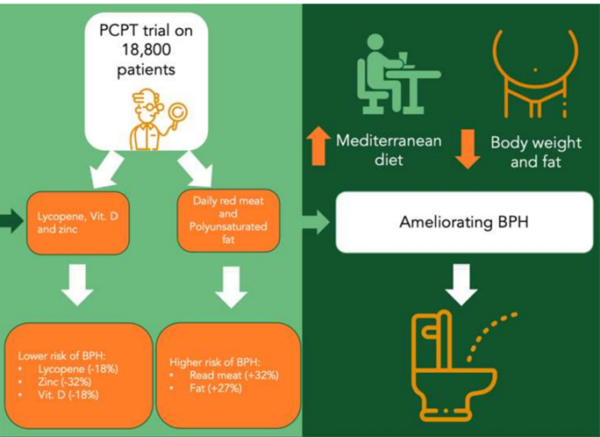
Among dietary influences, the overconsumption of red meat, fats and processed foods has been linked to an elevated risk of BPH. In contrast, diets abundant in vegetables and fruits have shown protective effects against BPH, likely due to their anti-inflammatory, antioxidant and hormone-modulating properties. For example, a 2008 re-analysis of data from the Prostate Cancer Prevention Trial, a landmark randomised clinical trial (RCT) involving 18,800 older men, revealed that (Figure 1):
- Men who consumed red meat daily had a 38% higher risk of BPH compared to those eating red meat less than once per week.
- Men who ate fewer than one serving of vegetables per day faced a 32% increased risk of BPH compared to those consuming four or more servings daily.
- Men whose diets were rich in lycopene, zinc and vitamin D experienced a 20-30% reduction in BPH risk.
More recent studies using the dietary phytochemical index (DPI) and dietary inflammatory index (DII) continue to highlight the role of diet in BPH. Briefly, DPI measures the intake of phytochemical-dense foods (e.g., whole grains, fruits, vegetables, nuts and legumes), whereas DII measures the inflammatory potential of a diet based on the intake of pro-inflammatory foods (e.g., processed meats, refined grains and added sugars). A 2024 study found that men with high DPI scores had a 70% lower risk of BPH compared to those with low scores. Conversely, another 2024 study reported that diets high in pro-inflammatory foods, reflected by elevated DII scores, were associated with a 30-40% increased risk of BPH.
Together, these findings reinforce the value of a plant-based diet, particularly one modelled after the Mediterranean diet, in preventing or slowing the progression of BPH (Figure 1). However, which specific plant extracts or compounds are responsible for these benefits of a plant-based diet, and what mechanisms underlie their effects?
Figure 1. Relationship between dietary patterns and benign prostatic hyperplasia (BPH), according to findings from the major Prostate Cancer Prevention Trial (PCPT). Source: Russo et al. (2021), Nutrients.
In 2021, the European Association of Urology (EAU) released a comprehensive clinical guideline for the management of male lower urinary tract symptoms, including BPH. For the first time, this guideline included a detailed review of the potential of plant extracts for BPH symptom relief. Such phytotherapy (phyto- means plant) approaches are typically not considered in standard medical practice, primarily because it does not align with the “one disease, one target, one drug” paradigm of modern medicine: e.g., statins target cholesterol enzymes in heart diseases, and antidepressants target neurotransmitters in mental disorders. In contrast, plant extracts often target multiple biochemical pathways simultaneously, making them harder to study within the framework of traditional drug development.
Among the reviewed plant extracts for BPH, the EAU recommended hexane-extracted Serenoa repens (HESr) due to its demonstrated efficacy and safety profile. For context, hexane extraction uses hexane as a solvent to concentrate the bioactive compounds, making the extract more potent. Also called saw palmetto, S, repens is a small palm tree native to the southeastern U.S. This EAU recommendation was informed by the following series of evidence:
- A 2009 meta-analysis of 30 randomised clinical trials (RCTs) found no significant benefits of repens compared to placebo for relieving BPH symptoms, improving urine flow or reducing prostate size. However, a key limitation of this meta-analysis is the inclusion of all brands of S. repens, regardless of the extraction methods.
- A 2016 meta-analysis of 12 RCTs focused on HESr only and found it was superior to placebo in reducing night-time urination and improving urine flow. Its effectiveness in relieving BPH symptoms was comparable to standard medications such as tamsulosin (α1-blocker) and finasteride (5α-reductase).
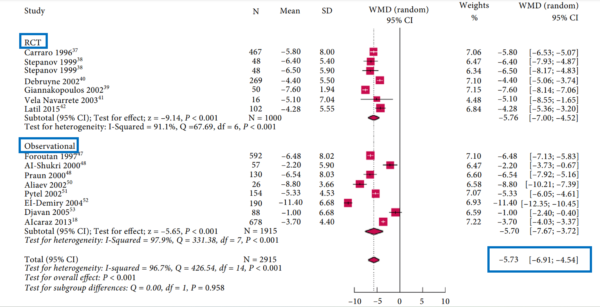
- A 2018 meta-analysis of 15 RCTs and 12 observational studies calculated that HESr decreased overall urinary symptoms by 5.7 points compared to placebo (Figure 2). Specifically, night-time urination was reduced by 1.6 fewer trips per night, urine flow improved by 2.9 mL per second, and prostate size shrunk by 7%. HESr also delivered similar symptom score improvements as tamsulosin or finasteride with significantly fewer side effects after six months of treatment. Mild gastrointestinal discomfort was the most common side effect of HESr, affecting only 3.8% of patients.
Meta-analyses combine data from multiple studies, usually RCTs, to draw more reliable conclusions. RCTs are the gold standard method in clinical research, providing the most reliable evidence for determining the efficacy of medical interventions. Based on this robust evidence showing that HESr confers comparable symptom relief to standard medications, the EAU recommends considering HESr for men with mild BPH who do not require urgent interventions and want to avoid treatment-related side effects. However, the EUA also takes a conservative stance, emphasising informing patients that, while HESr is safe and effective, its benefits may be modest compared to stronger pharmaceutical treatments.
Figure 2. Meta-analysis on the effects of hexane-extracted Serenoa repens (HESr) on overall urinary symptoms of BPH compared to placebo. Red squares represent individual data points from randomised clinical trials (RCTs) and observational studies. Red diamonds indicate the overall effect size of the synthesised data points, showing an improvement of −5.73 points. Source: Vela-Navarrete et al. (2018), British Journal of Urology International.
Interestingly, the EUA also cited a 2017 cohort study showing that combination therapy of HESr and silodosin (an α1-blocker similar to tamsulosin) provided meaningful symptom relief in 70% of men with BPH compared to just 30% of men in the silodosin-only group. Similarly, a 2014 RCT found that HESr and tamsulosin co-therapy was more efficacious at improving the clinical outcomes of BPH patients than tamsulosin alone. Hence, HESr could also be used as a complementary therapy to enhance the effectiveness of standard medications for BPH.
While Serenoa repens, particularly in its hexane-extracted form, remains the most robust and widely used phytotherapeutic agent for BPH, other plant extracts have also shown potential. Extracts from Pygeum africanum (African prune tree bark), Curcubita pepo (pumpkin seeds) and Solanum lycopersicum (tomatoes) have demonstrated positive effects in improving urinary symptoms of BPH in RCTs. Although the evidence for these plant extracts in BPH control is not as extensive as that for HESr, ongoing research continues to shed light on their promising roles in supporting prostate health.
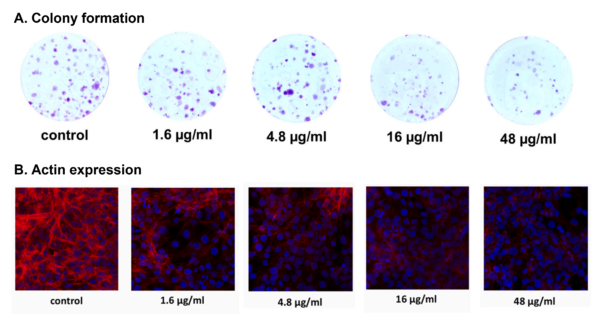
As clinical studies continue to confirm the efficacy and safety of plant extracts in managing BPH, particularly hexane-extracted S. repens (HESr), researchers have turned their attention to understanding the biological mechanisms behind its therapeutic effects. One 2022 study from the University Hospital of Munich, Germany, analysed how human prostate and bladder tissues respond to HESr. Results revealed that HESr reduces excessive muscle contractions in these tissues by blocking adrenergic and thromboxane receptors, which helps relieve pressure on the bladder. HESr also slows the growth of prostate cells by disrupting actin, a structural protein essential for maintaining cell shape. This prevents prostate cells from dividing and forming clusters, which could otherwise contribute to prostate enlargement (Figure 3). Importantly, HESr had no toxic effects on the human tissues, even at high doses.
Figure 3. Effects of hexane-extracted Serenoa repens (HESr) on human prostate cells. HESr effectively decreased the (A) colony formation and (B) actin expression of human prostate cells in a dose-dependent manner. Up to 50% and 80% reduction in colony formation and actin expression were seen at the highest dose of 48 µg/mL. Source: adapted from Tamalunas et al. (2022), Life Sciences.
Certain plant extracts also exhibit hormone-modulating properties important for controlling BPH. For example, S. repens and C. pepo (pumpkin seeds) can inhibit the activity of 5α-reductase, an enzyme responsible for converting testosterone into dihydrotestosterone (DHT), a hormone that promotes prostate enlargement. This mechanism mirrors the mechanism of finasteride, a common medication for BPH. On the other hand, P. africanum (African prune tree) can bind to and occupy androgen receptors, thus preventing androgenic hormones like DHT from stimulating prostate cell growth. By targeting these hormonal pathways, these plant extracts provide an additional layer of therapeutic benefit in BPH management.
Overall, these findings shed light on the mechanisms of plant extracts like S. repens, C. pepo and P. africanum in managing BPH. By targeting multiple pathways – such as relaxing smooth muscles, inhibiting abnormal prostate cell growth and modulating hormone activity – these plant extracts offer a multi-targeted approach to symptom relief.
Emerging evidence suggests that plant extracts also support broader aspects of men’s health, owing to their high content of antioxidant and anti-inflammatory phytochemicals. Studies have found that S. repens and P. africanum extracts can dampen inflammation in various conditions, such as obesity, pulmonary fibrosis and prostatitis (prostatic inflammation). For instance, a 2018 study reported lower infiltration of inflammatory cells in the prostates of men with prostatitis who were treated with hexane-extracted S. repens (HESr) compared to untreated patients. This finding bears wider implications because prostate inflammation also contributes to the development of other prostate diseases, including prostate cancer and BPH.
Phytotherapy is widely favoured for its safety profile. Standard medications for BPH (e.g., tamsulosin and finasteride) can lead to bothersome side effects, including erectile dysfunction, reduced libido and ejaculatory disorders. In contrast, phytotherapeutic agents like HESr had little to no impact on sexual function, as reported in the abovementioned 2018 meta-analysis. Notably, a 12-month RCT found that ejaculatory disorders occurred 7-times less frequently among users of HERs than tamsulosin. This superior safety profile is attributed to the multi-targeted action of HESr, which further modulates inflammatory and hormonal activities without impairing smooth muscle function in penile tissues.
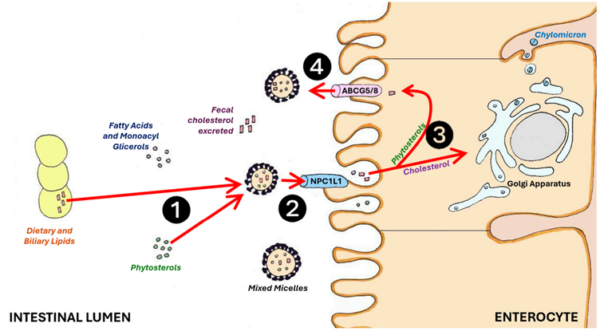
Beyond prostate health, plant extracts like S. repens, C. pepo and P. africanum also contribute to cardiovascular health. These extracts are abundant in phytosterols, a class of phytochemicals structurally similar to human cholesterol. This structural similarity enables phytosterols to compete with dietary cholesterol for absorption in the intestines, thus reducing the levels of low-density lipoprotein (LDL) cholesterol (Figure 4). As elevated LDL cholesterol is a risk factor for cardiovascular diseases, phytosterols play a critical role in promoting heart health.
Overall, these findings align with a robust body of evidence that a phytochemical-rich, plant-based diet promotes longevity and helps prevent a wide range of chronic diseases, including prostatic conditions like prostatitis, prostate cancer and BPH. By integrating these plant extracts into BPH management, we adopt a holistic approach that goes beyond symptom relief, supporting prostate health while enhancing long-term vitality for men.
Figure 4. How phytosterols prevent dietary cholesterol absorption in the intestines. Phytosterols (1) compete with cholesterol for absorption in the intestines by binding to the NPC1L1 protein (2). Once absorbed, free phytosterols (3) are sent back into the intestinal lumen by ABCG5/8 transport proteins in the intestinal cells (4), promoting the removal of cholesterol through the faeces (shown by red arrows). Abbreviations/acronyms: ABCG5/8, adenosine triphosphate–binding cassette transporter G5/8; NPC1L1, Niemann-Pick C1-Like 1. Source: Fogacci et al. (2024), Nutrients.