New Evidence Says Yes, If We Act Early
Radiotherapy has long been a cornerstone of prostate cancer treatment. While the technology has advanced, the burden of side effects remains. Radiation is inherently toxic, after all, which scientists have managed to harness to kill cancer cells. But what if we could predict and even prevent the long-term complications by paying closer attention to what happens in the first few months after radiotherapy? A new meta-analysis suggests we can, which sheds light on a window of opportunity: the moment acute side effects begin. What we do during this critical period might make all the difference in a patient’s long-term health.
Used in more than half of all cancer cases worldwide, radiotherapy plays an established role in controlling tumours and improving survival. Over the past century, radiotherapy has evolved dramatically in terms of precision. Yet, even the most sophisticated techniques cannot completely eliminate exposure of normal tissues to radiation. This unavoidable collateral dose can lead to adverse side effects, which are often divided into acute and late toxicity.
Acute side effects show up during or within three months after radiotherapy. In prostate cancer radiotherapy, acute toxicity tends to affect fast-growing healthy tissues in the bladder lining or bowel wall. These areas rely on stem cells to constantly renew themselves. However, radiation can damage these stem cells and cause symptoms like urinary urgency, bowel discomfort or inflammation. If the damage is mild, the tissue usually repairs itself within a few weeks, and symptoms will resolve, but this is not always the case.
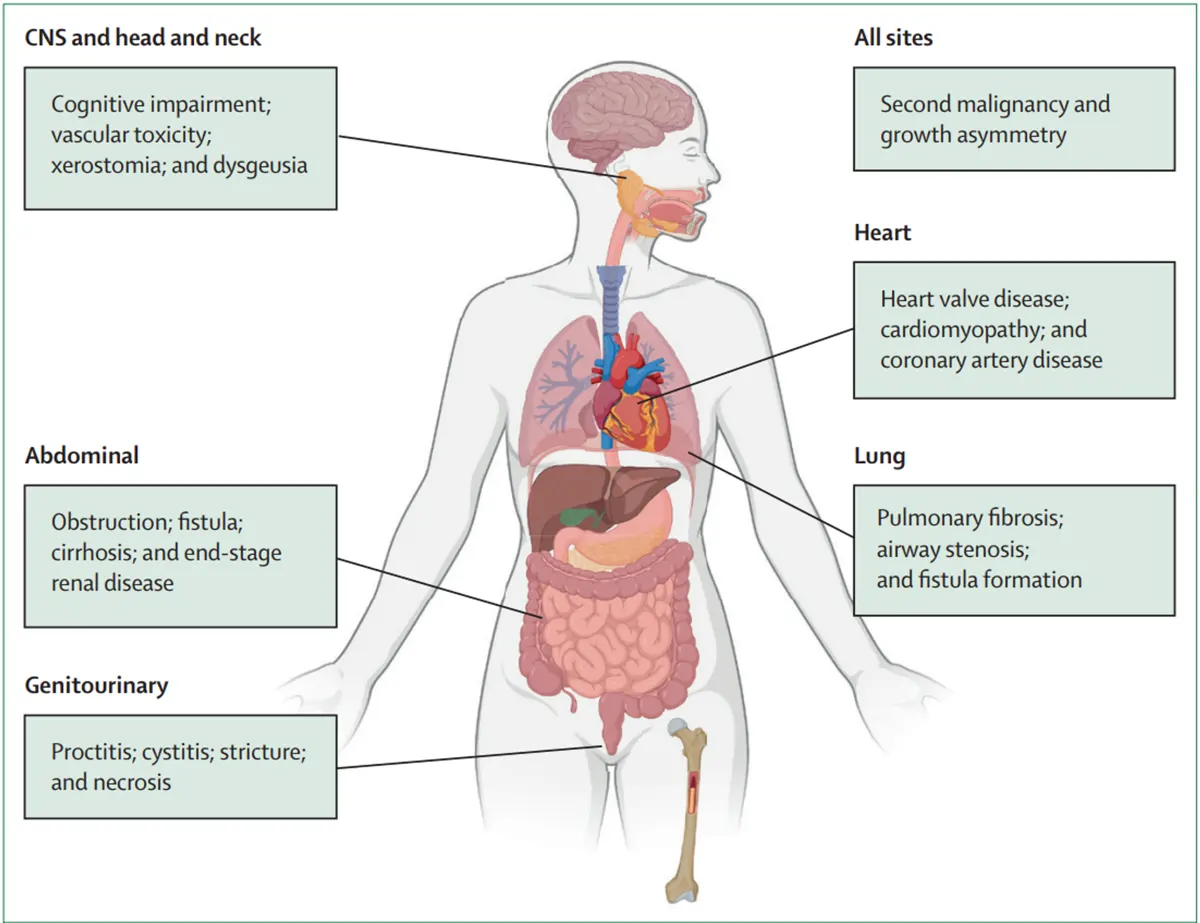
If the body does not fully heal, especially when radiotherapy is combined with chemotherapy or therapies that make it harder for tissues to recover, those early injuries can turn into long-term problems. Such late toxicity arises because radiation can damage slow-repairing tissues (e.g., blood vessels, fat, muscle and connective tissue), resulting in long-term biological changes like fibrosis (scarring) and chronic inflammation. These complications are often progressive and impair organ function over time, which can be irreversible. Depending on the treatment site and radiation dose, a wide range of long-term toxic effects have been reported across multiple organ systems (Figure 1).
In prostate cancer radiotherapy, late toxicity typically affects the genitourinary (genital and urinary) and gastrointestinal systems. Common urinary problems include increased frequency, urgency, pain during urination and, in some cases, incontinence or bleeding from the bladder – a condition known as radiation cystitis. Some patients may also develop urethral strictures, where scarring narrows the urinary passage and makes it difficult to pass urine. The genital-related side effects include erectile dysfunction, which can develop when radiation damages blood vessels and nerves involved in erections.
Figure 1. General overview of the most common long-term toxic effects of radiotherapy in different sites of the body. Source: Verginadis et al. (2025), The Lancet.
On the gastrointestinal side, radiation can damage the rectum and bowel, leading to radiation proctitis with symptoms of chronic diarrhoea, rectal bleeding, and bowel urgency, as well as pain during bowel movements. In rare cases, radiation can lead to ulcers in the bowel and, in more severe situations, a fistula – an abnormal tunnel-like connection between the rectum and bladder that can leak stool or urine.
Understanding and tackling radiotherapy toxicity is paramount for improving long-term outcomes in prostate cancer care. While advancements in radiotherapy have greatly reduced some risks, late complications remain a real concern. This is particularly important for prostate cancer patients who are diagnosed at an early stage and can expect to live for many years after treatment. As a result, even modest long-term side effects can accumulate and downgrade the quality of life over time. Therefore, it is vital that we identify patients who are most at risk and find ways to intervene before lasting damage sets in.
For decades, the acute and late toxicity phases of radiotherapy were seen as separate entities: Acute side effects are temporary and affect fast-growing tissues, while late effects are chronic (long-lasting) and affect slow-repairing tissues. However, new research is challenging this view, suggesting that early side effects may be warning signs for long-term harm. In this light, the early weeks of radiotherapy may represent a critical window: one that could allow clinicians to anticipate and perhaps even prevent the long-term complications.
The new research, published in The Lancet Oncology, was led by Nikitas et al. from institutions across the U.S., U.K., Netherlands, Italy and Canada. In this international effort, Nikitas et al. conducted a comprehensive meta-analysis using individual patient data, which re-analyses patient-level data from multiple studies for greater accuracy. A total of six phase III randomised clinical trials (RCTs) investigating prostate radiotherapy were pooled in the meta-analysis. As the gold standard in clinical research, RCTs work by randomly assigning participants to the experimental or control groups. Randomisation equally distributes all variables between the two groups, ensuring that the results observed are strictly due to the experimental intervention alone rather than other factors.
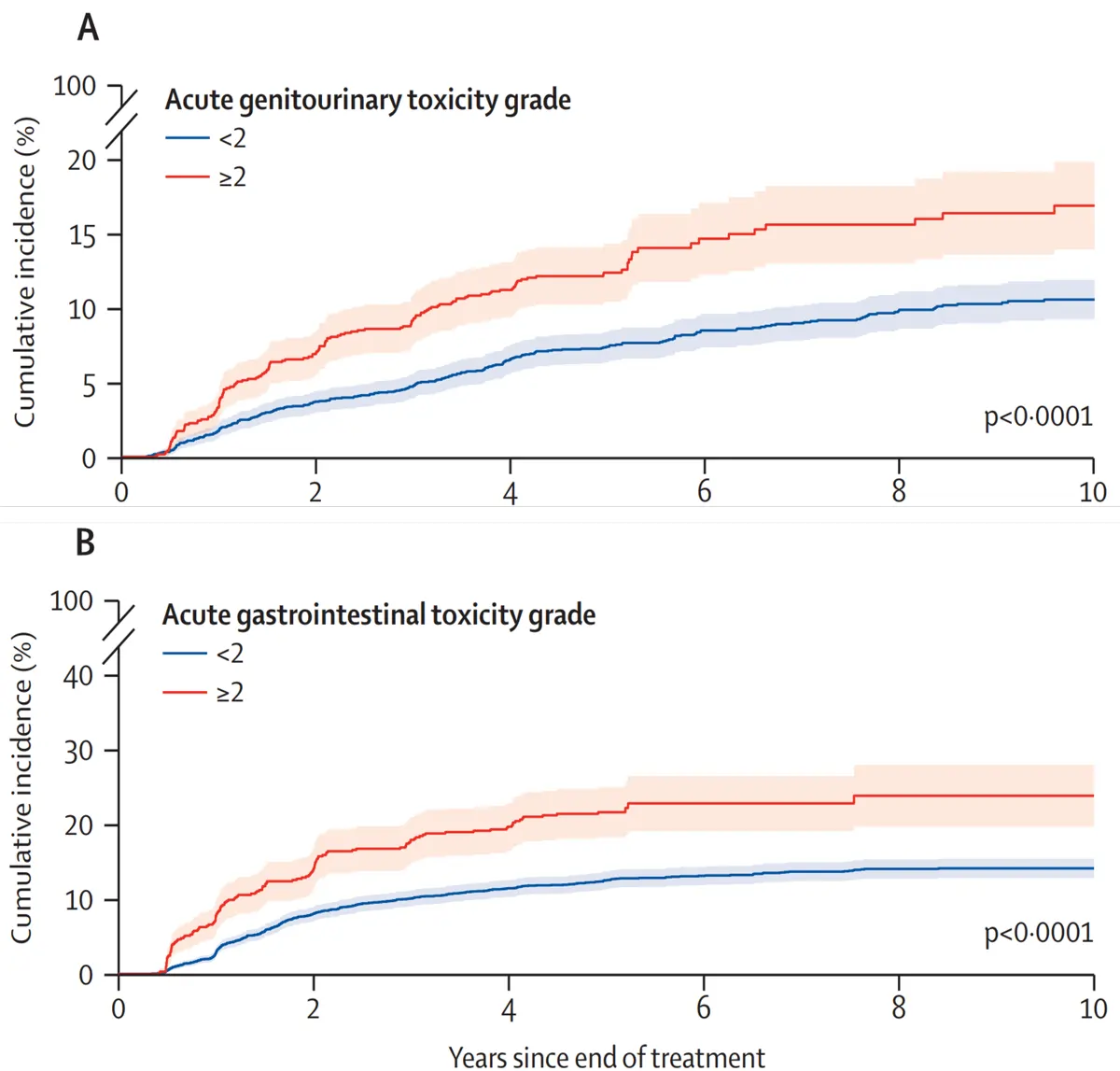
Pooling data from the six RCTs provided a total sample size of 6,593 prostate cancer patients treated with radiotherapy, with a median age of 69 years and a follow-up duration of six years. After analysing the pattern of radiotherapy toxicity over time, Nikitas et al. uncovered a clear and consistent pattern: patients who experienced moderate or worse side effects soon after radiotherapy were more likely to develop long-term problems (Figure 2):
- Urinary Side Effects: Men who had moderate or worse urinary problems during the first three months after radiotherapy were 2.2-fold more likely to develop long-term urinary complications. Nearly one in five men had a drastic drop in urinary quality of life, and over 65% of patients with late toxicity did not recover at 7-year follow-up.
- Bowel Side Effects: Men who experienced moderate or worse bowel symptoms during the first three months after radiotherapy were 2.5-times more likely to develop long-term bowel issues. About 1 in 3 patients reported a substantial decline in bowel-related quality of life, and over 57% did not recover at 7-year follow-up.
In the overall study population, 15.4% of men developed moderate or worse urinary side effects, and 14.7% experienced similar bowel complications in the long term. Most of these late effects appeared more than six months after treatment. While factors such as higher radiation doses and hypofractionated schedules (i.e., fewer sessions with stronger doses) were also linked to a greater risk of late toxicity, acute side effects remained the strongest and most consistent predictor of long-term harm, regardless of treatment regimen.
Figure 2. Cumulative incidence curves for late grade ≥2 genitourinary (A) and gastrointestinal (B) toxicity. Toxicity was graded using standard clinical criteria (RTOG/EORTC and CTCAE), where grade 1 is mild, grade 2 is moderate, grade 3 is severe, grade 4 is life-threatening and grade 5 is death. As shown, patients who experienced moderate or worse acute side effects (grade ≥2) were about twice as likely to develop late toxicity compared to those with only mild or no early symptoms. Source: Nikitas et al. (2025), The Lancet Oncology.
“To our knowledge, this [meta-analysis] provides the best evidence to date supporting the phenomenon of consequential late toxicity following radiotherapy in the context of prostate cancer,” the authors wrote. They further emphasised that the connection between early and late side effects could offer a valuable chance to step in. If clinicians can recognise and respond to acute symptoms early enough, they may be able to change the course of a patient’s recovery and prevent those symptoms from turning into long-term complications.
“These results show that acute toxicities following prostate radiotherapy are associated with late toxicities months and years later,” Dr John Nikitas, lead author of the study and a radiation oncology specialist at the University of California, said in a news report. “This underscores the importance of measures that reduce the risk of acute toxicities because they may also potentially improve long-term outcomes and quality of life for patients.”
The recognition that acute toxicity may foreshadow long-term harm shifts the way clinicians should think about radiotherapy side effects. This insight opens a critical window of opportunity: the first few weeks to months after radiotherapy, when proactive intervention could make all the difference in the long-term outcome of patients.
Current management strategies include early and consistent symptom monitoring during radiotherapy. Regular assessments with the radiation oncology team allow clinicians to detect and address emerging symptoms before they escalate. Anti-inflammatory medications and dietary adjustments may also be used as necessary to facilitate the healing of inflamed tissues. Early management of treatment-related side effects can also improve adherence and ultimately enhance long-term results. Researchers have also recommended the “selection of patients for radiotherapy,” where radiotherapy may be contraindicated (medically inappropriate) for patients with underlying conditions that impair tissue healing, such as autoimmune disorders affecting connective tissues or genetic syndromes affecting DNA repair.
Furthermore, cutting-edge innovations in radiotherapy technologies now allow clinicians to adjust therapy in real time and minimise toxicity from the start. Specifically, these innovations aim to precisely target cancer cells while sparing surrounding healthy tissue, which would decrease the likelihood of acute damage and, by extension, late toxicity:
- Adaptive image-guided radiotherapy: It uses daily computed tomography (CT) or magnetic resonance imaging (MRI) to track tumour movement in real time, allowing radiotherapy to be precisely adjusted to the tumour’s position each day.
- Proton therapy: Unlike traditional radiotherapy relying on photons like X-rays or gamma rays, which deposit radiation energy along the entire path through the body, proton beams release most of their energy directly at the tumour site.
- Stereotactic radiotherapy: Delivers high-dose radiation from multiple angles in fewer, highly focused sessions, which limits radiation to surrounding tissues.
- Brachytherapy: Places a radioactive source inside or next to the tumour. As the radiation source only travels a short distance, it effectively targets the tumour while sparing nearby healthy tissues.
- Rectal spacers: In prostate cancer radiotherapy, gel-like materials are inserted between the rectum and prostate to physically distance the rectum from the high-dose radiation zone. By reducing the radiation exposure to the rectum, this technique helps minimise bowel or gastrointestinal toxicity.
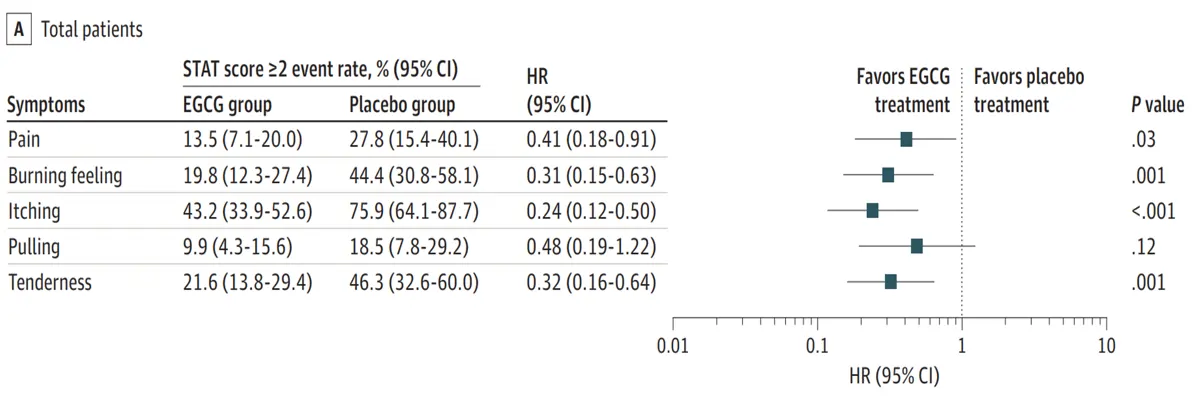
Notably, there is also a growing interest in complementary strategies to provide additional protection during this early phase. For instance, the use of plant-based compounds with anti-inflammatory, antioxidant and wound-healing properties can mitigate acute toxicity, support tissue recovery and prevent long-term complications. Emerging clinical research has reported that plant compounds like curcumin (from turmeric), epigallocatechin-3-gallate (ECGC; from green tea), and a combination of resveratrol, lycopene, vitamin C and anthocyanin can reduce the incidence and severity of the acute side effects of radiotherapy (Figure 3).
Overall, these findings represent a pivotal shift in mindset: from reacting to late toxicity to proactively preventing it during the early phase. Recognising the acute phase as a gateway to long-term outcomes allows clinicians and patients to take timely and targeted action. By combining symptom monitoring, advanced radiotherapy technologies and personalised treatment plans, we can move towards a better standard of care that not only treats cancer but also protects the future well-being of survivors.
Figure 3. Green tea EGCG reduced radiation-induced inflammatory symptoms of grade 2 or greater in a clinical trial of breast cancer patients. Specifically, EGCG treatment reduced symptoms of pain, burning feeling, itching and tenderness compared to placebo. Although this study examined breast cancer, it still offers valuable insights, as breast and prostate tissues both share similar risks of acute inflammatory damage during radiotherapy. Source: Zhao et al. (2022), JAMA Dermatology.