The Gut Immune System
The gut is not only a vital digestive organ but also the body’s largest immune hub, harbouring about 70-80% of our immune cells. Such a vast immune resource is dedicated to the gut because it needs to surveil trillions of microbes in the gut microbiota, alongside potential pathogens and carcinogens we ingest. This creates a dynamic battleground where the gut immune system constantly interacts with the microbiota and external environment, always preparing to neutralise foreign invaders. Scientists analogise this to, “To make peace, prepare for war.”
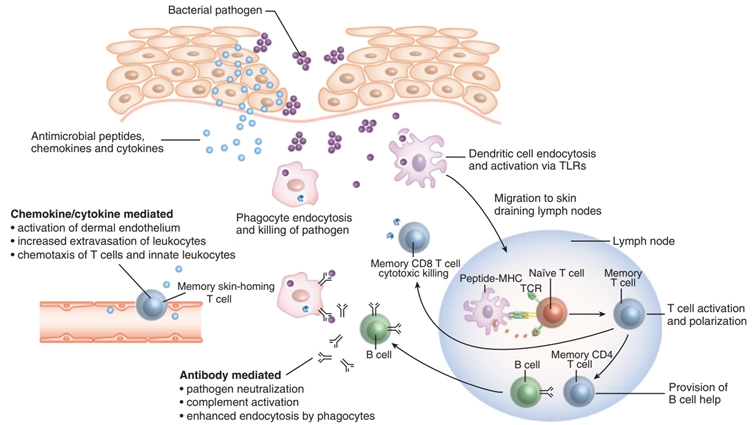
Before we proceed, a brief concept of immunology is recommended. When a foreign threat like a pathogen appears, the innate immune system first launches generalised inflammatory responses and phagocytes to engulf the pathogen. Next, dendritic cells present a fragment of the pathogen to activate B-cells and T-cells of the adaptive immune system. B-cells then produce antibodies to neutralise the pathogen, while T-cells inject cytotoxic substances to eradicate the threat (Figure 1). B-cells and T-cells develop immunological memory of the pathogen, facilitating more rapid and effective responses during subsequent encounters with the same pathogen. Thus, the immune system has two main branches: the first-line, generalised innate response and the second-line, more precise but slower adaptive response.
Focusing on T-cells, they serve two primary functions as helper and killer T-cells. The former enhances the activities of other immune cells, whereas the latter injects cytotoxins into abnormal cells, such as infected or cancerous cells. Hence, T-cells form an indispensable part of anti-cancer immunity. Most types of T-cells, present in the blood and lymphoid organs (e.g., lymph nodes and spleen), belong to the alpha-beta subset. However, a rarer subset of gamma-delta T-cells also exist, which are found predominantly throughout the gut lining. This newsletter dives into the anti-cancer capacity of gamma-delta T-cells and discusses how their efficacy can be reinforced through certain complementary medicine strategies.
Figure 1. How the immune system responds when a foreign threat, such as a bacterial pathogen, invades the body via the skin or other body parts. First, the innate immune system deploys a generalised response, consisting of antimicrobial peptides and pro-inflammatory chemokines and cytokines. It also deploys phagocytes to engulf and kill the pathogen, as well as dendritic cells to migrate to the lymph nodes to activate B-cells and T-cells of the adaptive immune system by presenting an antigenic piece of the pathogen. B-cells secrete antibodies, while T-cells mediate cytotoxic killing of the pathogen. Source: Clark and Kupper (2005).
The Anti-cancer Capacity of Gamma-delta T-cells
Gamma-delta T-cells, surprisingly, exhibit features of both the innate and adaptive immune systems. They can generate rapid, generalised immune responses without being activated by dendritic cells, making them highly vigilant in surveillance of any infection or cancer. As a result, gamma-delta T-cells may have been evolutionarily poised to be situated throughout the gut lining with maximal exposure to the external environment. Yet, akin to adaptive immune cells, gamma-delta T-cells can undergo self-replication to generate an army of specialised immune cells to attack the targeted threat and form long-term memory of the threat.
The innate immune attributes of gamma-delta T-cells are a fascinating yet underappreciated ability, as it indicates that such T-cells can recognise and attack a broad range of infections and cancers. In contrast, conventional alpha-beta T-cells only recognise one unique antigen, limiting their target to one specific threat. Scientists are, therefore, excited to harness the power of gamma-delta T-cells as a universal cancer immunotherapy.
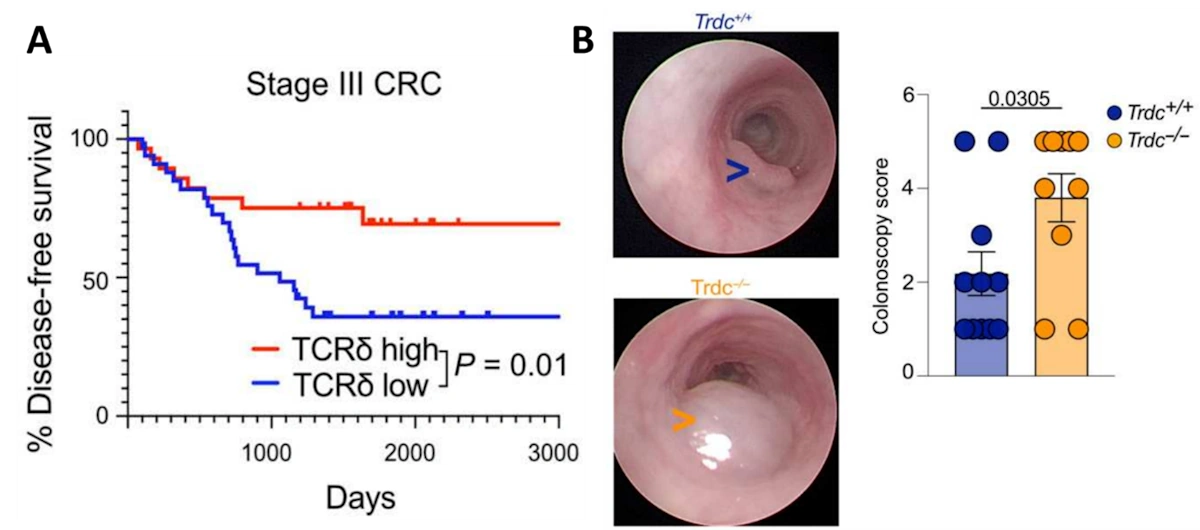
As gamma-delta T-cells are mainly found in the gut, their anti-cancer effects against colorectal tumours have been well examined. One landmark study of patients with advanced colorectal cancer found that gamma-delta T-cells in the tumour site correlated with improved survival rates (Figure 2A). The study also showed that colorectal tumours grew more rapidly in mice deficient in gamma-delta T-cells (Figure 2B). Subsequent experiments revealed that the anti-cancer effects of gamma-delta T-cells stem from the production of granzyme B and lymphotactin. Granzyme B is a cytotoxin that induces apoptotic cell death in targeted cells, while lymphotactin is a chemokine that attracts other T-cells to the foreign threat.
Figure 2. The importance of gamma-delta T-cells in colon cancer. (A). Disease-free survival rates of patients with stage III colorectal cancer, separated by those with high (red) and low (blue) numbers of tumour-infiltrating gamma-delta T-cells (TCRδ). (B). Colonoscopy images and size scoring of the colorectal tumour in mice with (Trdc+/+, blue) and without (Trdc−/−, yellow) gamma-delta T-cells. Source: Yakou et al. (2023), Science Immunology.
Beyond the gut, gamma-delta T-cells also circulate in the bloodstream in small amounts to mediate anti-cancer response against other cancers. Recognising their potential, scientists have attempted blood infusion of gamma-delta T-cells as a form of cancer therapy. This approach has been successfully performed in several phase I clinical trials of patients with solid tumours, showing positive signs of clinical improvement without any treatment toxicity. These gamma-delta T-cells were harvested from the blood of healthy donors and subsequently cultured and replicated in the laboratory. As these T-cells were derived from a different person, the therapy is called allogeneic (genetically distinct) gamma-delta T-cell therapy.
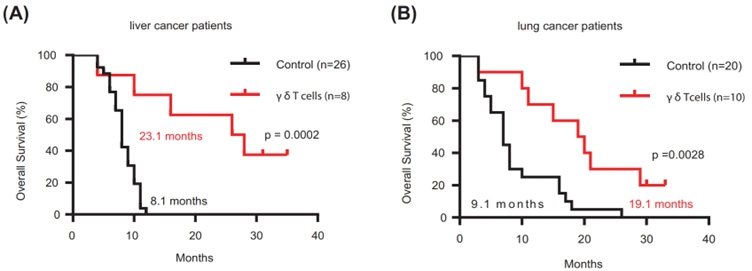
Further phase II trials have reported that gamma-delta T-cell therapy was highly efficacious at improving the clinical outcomes of cancer patients. For instance, one of the trials demonstrated that patients with advanced liver or lung cancer who received this therapy were still alive at 19-23 months during follow-up, but their control counterparts who did not receive the experimental therapy died at 8-9 months (Figure 3). Due to the novelty of this therapy modality, however, phase III trials are currently in progress, and no gamma-delta T-cell therapy has received approval from the Food and Drug Administration (FDA).
Despite its promising potential, gamma-delta T-cell therapy still faces certain obstacles. For one, its broad anti-cancer spectrum also reduces its precision. One clinical trial showed that only small numbers of gamma-delta T-cells managed to penetrate the tumour site, warranting more research into enhancing their tumour-infiltrating capacity. Second, cell culture conditions may differ between laboratories, resulting in inconsistent replication and production of gamma-delta T-cells. Third, gamma-delta T-cells have multiple subtypes in varying proportions, necessitating further study to determine the most effective subtype combinations for specific cancers. Regardless, with adequate scientific efforts, overcoming these challenges to fully realise the potential of gamma-delta T-cells as cancer therapies is possible.
Figure 3. Overall survival of patients with advanced liver or lung cancer treated with allogeneic gamma-delta (γδ) T-cells. (A) For liver cancer, the median survival time of treated patients (red line) was 23.1 months versus 8.1 months in untreated patients (black line). (B) For lung cancer, the median survival time of treated patients (red line) was 19.1 months versus 9.1 months in untreated patients (black line). These differences were statistically significant (p < 0.001 and p < 0.01, respectively). Source: Xu et al. (2021), Cellular and Molecular Biology.
Complementary Strategies to Enhance Gamma-delta T-cells
Although gamma-delta T-cell therapy may not receive clinical approval soon, complementary strategies can boost the performance of these rare T-cells. Complementary therapy involves holistic treatments, such as natural compounds in the form of supplements, designed to complement the effectiveness of standard medical interventions. The Pfeifer Protocol, for instance, strategically capitalises on the inherent anti-cancer and immune-modulating properties of selected plant compounds to improve patient outcomes. Excitingly, several of these compounds have been found to bolster the activities of gamma-delta T-cells:
- A Japanese clinical trial showed that resveratrol, a compound in grapes and berries, supplementation increased the population of anti-inflammatory regulatory T-cells and gamma-delta T-cells in the blood of healthy individuals (Figure 4A). Additionally, a decrease in the levels of pro-inflammatory mediators was observed in these individuals (Figure 4B).
- A Chinese pre-clinical study demonstrated that quercetin, a flavonoid commonly found in plants, enhanced the activities of gamma-delta T-cells in triggering apoptotic cell death in cancerous and even pre-cancerous cells of the human breast tissue.
- An American clinical trial found that the intake of dried shiitake mushroom promoted the proliferation of gamma-delta T-cells and mitigated pro-inflammatory processes in the immune system of healthy adults.
In summary, gamma-delta T-cells are a rare subset of T-cells primarily located in the gut, with small amounts circulating in the blood. As they form a crucial part of anti-cancer defence, scientists are starting to explore the feasibility of gamma-delta T-cell therapy in cancer. While this novel therapy modality may not be immediately available for clinical use, there are existing complementary methods to improve the functionality of gamma-delta T-cells to augment our body’s natural anti-cancer defence. Armed with this knowledge, you can adopt these complementary strategies to safeguard your health against diseases like cancer.