Neuro-Oncology: Harnessing the Nervous System to Combat Cancer
Introduction
The nervous system’s role in controlling and coordinating the body’s physiology is nothing short of magnificent. It uses electrical signals to control not only voluntary movements but involuntary processes as well, such as heart and breathing rates, digestion and even immunological functions. Neurons are the building blocks of the nervous system, which extend to almost every tissue and organ in the body, including those that turn cancerous. Thus, it is unsurprising that neurons can influence the initiation, growth and spread of cancer. This is the field of cancer neuroscience or neuro-oncology.
In this newsletter, we will delve into the three primary mechanisms of the nervous system-cancer interactions, followed by how these mechanisms can be leveraged to combat cancer.
The Main Mechanisms of Neuron-Cancer Crosstalk
The existence of neuro-oncology was first hinted when two studies independently discovered that breast cancer cells expressed high levels of β-adrenergic receptors in 1989 and 1990. β-adrenergic receptors respond to epinephrine or norepinephrine, which govern sympathetic (fight-or-flight) activities. A subsequent study showed that persistent stimulation of adrenergic receptors resulted in gastric cancer in rats. In the 2000s, more studies replicated this finding with other cancer types, showing that norepinephrine can activate biochemical pathways that promote angiogenesis.
Angiogenesis is the formation of new blood vessels, which allows more oxygen and nutrients to be delivered to the tumour. This process enables the tumour to grow and provides a pathway for cancer cells to enter the bloodstream and spread elsewhere. Cancer cells exposed to norepinephrine have been found to exhibit increased levels of vascular endothelial growth factor and matrix metalloproteinases, which are proteins that drive angiogenesis.
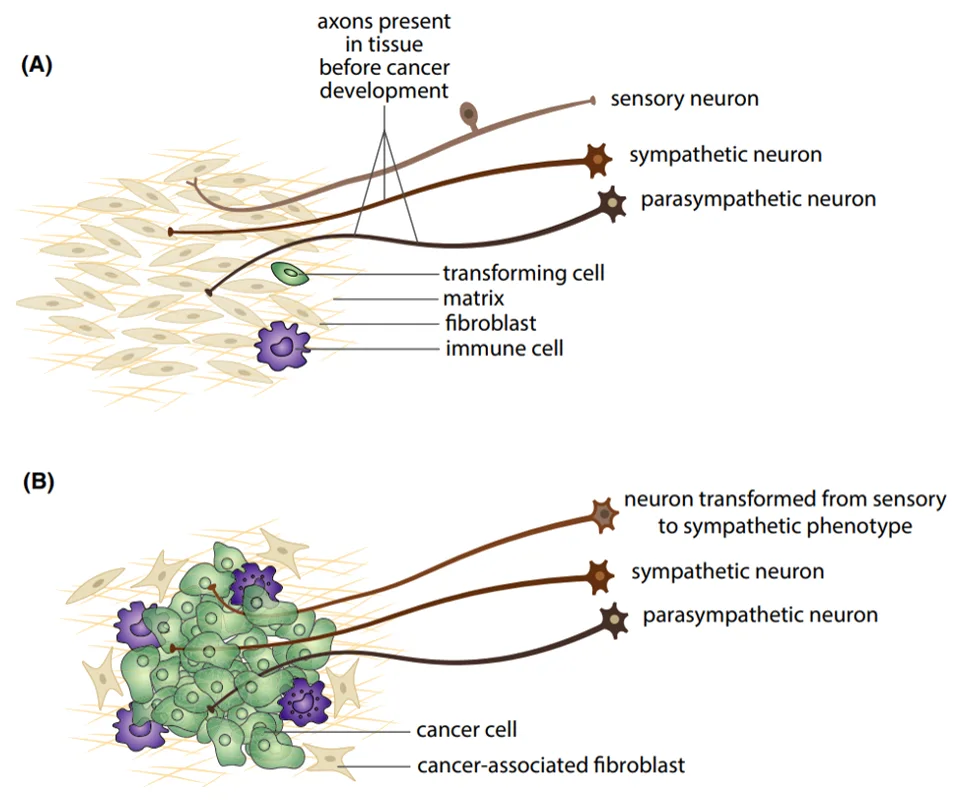
However, one question remains: What triggers the enhanced adrenergic signalling to the tumour or cancer cells? It was not until 2020 that a study answered this enigma, showing that the loss of TP53 in tumours induced neuronal differentiation into the adrenergic phenotype. TP53 is a gene that encodes the p53 protein, which regulates cell replication and neuron regeneration. Over time, neurons innervating TP53/p53-deficient tumours would lose their regulatory signals and transform into adrenergic neurons that respond to norepinephrine, facilitating angiogenesis (Figure 1).
Figure 1. Adrenergic neuronal transformation in cancer cells. (A) Typical sympathetic (adrenergic, fight-or-flight response), parasympathetic (cholinergic, rest-and-digest response) and sensory neurons in normal tissues. (B) Sensory neurons can transform into sympathetic, adrenergic phenotype during cancer development. Source: Mravec (2022), Cancer Medicine.
Besides angiogenesis, adrenergic signalling in cancer cells also poses other detrimental effects. For example, a 2007 study first demonstrated that even short-term exposure to stress chemicals, including norepinephrine, significantly increased DNA damage and interfered with DNA repair activities in cells. Further studies showed that norepinephrine also triggers DNA damage in breast, ovarian and other cancer cells, often resulting in accelerated cancer progression.
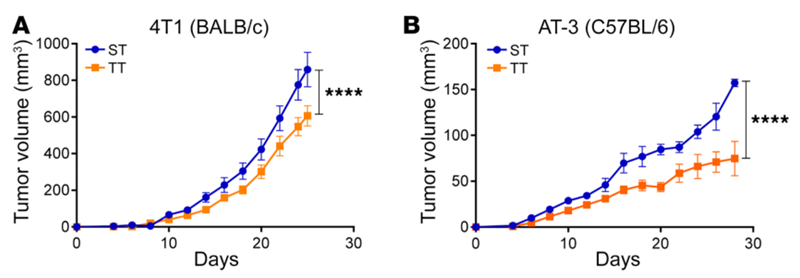
It is also widely known that stress compromises the immune system, increasing the susceptibility to illnesses. This is further exemplified when studies showed that norepinephrine inhibited the release of tumour necrosis factor-alpha (TNF-α) from cancer cells, thereby limiting the activities of T-cells. TNF- α is an inflammatory cytokine with various functions, including mediating tumour apoptosis (programmed cell death) and T-cell activation. As part of the adaptive immune system, T-cells eliminate cells deemed as foreign threats, such as cancerous cells. Moreover, an animal study showed that chronic stress activated adrenergic signalling to suppress anti-cancer immune responses, causing breast tumour growth (Figure 2).
Overall, the mechanisms of neuron-cancer crosstalk mainly centre on the effects of norepinephrine. This stress neurochemical can activate adrenergic receptors to stimulate tumour angiogenesis, induce DNA mutation and suppress anti-cancer immune activities. Consequently, targeting adrenergic signalling may open new therapeutic avenues in cancer care.
Figure 2. Tumour growth in mice bearing breast tumour cells, housed at sub-thermoneutral (ST, 22°C) or thermoneutral (TT, 30°C) temperatures. BALB/c and C57BL/5 represent different mice species, while 4T1 and AT-3 are different types of breast cancer cells. Mice housed in colder ST conditions had significantly increased tumour growth compared to normal TT conditions (P < 0.0005, denoted by ****). Source: Mohammadpour et al. (2019), Journal of Clinical Investigation.
Capitalising on the Neuron-Cancer Crosstalk for Therapeutic Purposes
When studies explored the pro-cancer effects of adrenergic signalling, many also evaluated the anti-cancer effects of adrenergic receptor blockers, most commonly propranolol. This approach helps to solidify cause-and-effect by showing that blocking adrenergic signalling can counteract their cancer-promoting effects.
Propranolol is the preferred choice because it was the first β-adrenergic receptor blocker approved for treating coronary artery disease and hypertension. As a result, propranolol has a long history of clinical safety and efficacy data. Indeed, several studies found that patients who happened to be on propranolol medication have significantly lower rates of cancer development and progression. This phenomenon has been observed with pancreatic-, breast-, prostate- and many other cancers.
Research also indicates propranolol can enhance the effectiveness of existing cancer therapies. For instance, two randomised clinical trials (RCTs) showed that propranolol administration alongside surgery decreased tumour-related activities in breast cancer patients, contributing to improved clinical outcomes over the placebo group. RCTs are the gold standard in medical research, possessing the capacity to influence healthcare policies. However, the use of adrenergic receptor blockers like propranolol in cancer care is relatively novel and still under investigation, so they are not yet part of standard cancer therapy protocols.
Nonetheless, it is wise to actively mitigate stress responses in our bodies. Stress accelerates ageing and, consequently, age-related diseases like cancer. This is where the role of complementary medicine becomes significant, exemplified by approaches like the Pfeifer Protocol that utilises potent anti-cancer plant compounds. These plant compounds act holistically by modulating the oxidative stress and immune responses in the body.
Interestingly, some of these plant compounds also can suppress adrenergic signalling, reinforcing their anti-cancer potential:
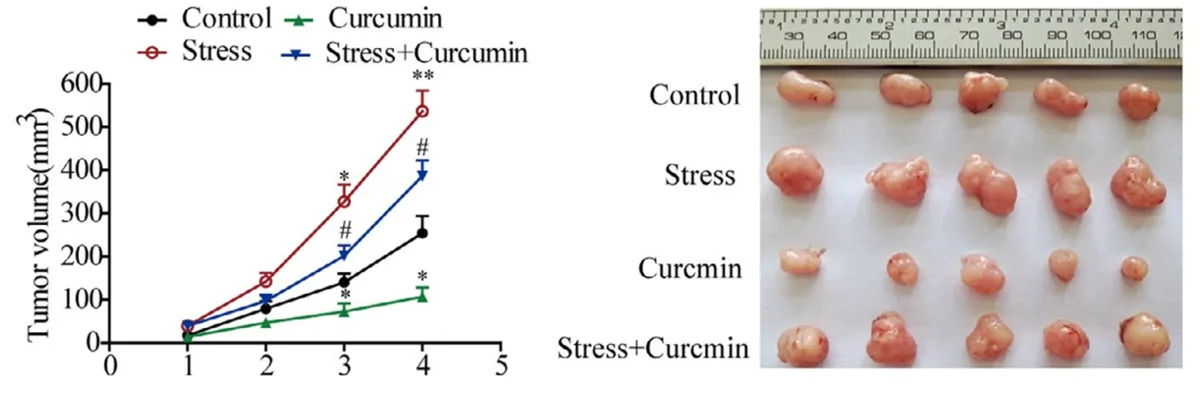
- Curcumin: A 2021 study used norepinephrine to stimulate the growth of brain tumours in mice, but curcumin administration prevented this effect (Figure 3).
- Quercetin: A pair of studies found that quercetin inhibited adrenergic signalling to suppress the spread and invasiveness of breast cancer cells.
Resveratrol: A 2018 study showed that resveratrol managed to induce apoptosis in cancer cells grown under norepinephrine-induced stress conditions. Resveratrol achieved this by downregulating the expression of β-adrenergic receptors on cancer cells.
Figure 3. Curcumin inhibited the growth of brain tumour in mice. Left: Tumour volume was greatest in the norepinephrine-induced stress condition (red), which was reduced significantly with curcumin administration (blue). Curcumin alone (green) also reduced tumour growth compared to control without intervention (black). Right: Visualisation of brain tumour volume in different conditions. Source: Wang et al. (2021), Journal of Cellular and Molecular Medicine.
Conclusion
Excessive and chronic stress, both mental and physical, is known to drive age-related diseases, including cancer. This is mainly due to the vast network of the nervous system, which innervates nearly every organ and tissue in the body, including tumours. As a result, stress neurochemicals like norepinephrine have been found to activate adrenergic neural pathways that promote cancer development and growth.
It then becomes crucial to adopt measures that minimise the detrimental biochemical responses of stress in our lifestyle, be it through adequate sleep, exercise, or nourishment. As part of a healthy diet, incorporating bioactive plant compounds with known abilities to suppress adrenergic signalling could be a key strategy in thwarting cancer and other ageing-related diseases.