The Immunome: An Introduction
In June 1999, Dr Thoru Pederson, a professor of cell biology at the University of Massachusetts Medical School, U.S., attended an academic conference in Oslo, where scientists shared their preliminary and major discoveries. The conference was dedicated to immunology topics, especially autoimmune diseases. Although Dr Pederson was not an immunology expert, his observations would later give rise to the field of the immunome.
“Although I was an invited speaker (on ribonucleoprotein autoantigens), I am an immunology Ausländer [German for foreigner],” Dr Pederson wrote in a 1999 paper. “I was struck by how intellectually intractable the phenomenon of autoimmunity has remained these five decades.”
In particular, Dr Pederson was impressed by the sheer number of antibody and antigen receptor genes that can be rearranged and recombined in infinite ways to create a highly diverse and adaptable immune response. This versatility enables the immune system to combat any kind of foreign invaders, whether pathogens, toxins, allergens, cancer cells or, unfortunately, transplanted organs. As a result, genetic data on the immune system burgeoned, prompting Dr Pederson to coin the term ‘immunome’ to encapsulate this expansive dataset in his 1999 paper.
In biology, the suffix “-ome” denotes the complete set of elements that make up a biological system. It originated from the word “genome,” which refers to all genes in an organism. This suffix has been extended to include the proteome (all proteins), metabolome (all metabolites), lipidome (all lipids), microbiome (all microbes) and, more recently, immunome (all immune components). Efforts to characterise the immunome have identified as many as 847 essential genes and proteins, whose activities vary among individuals due to differences in health status, genetics and lifestyle choices. In this sense, the immunome is unique to each person. The conceptualisation of the immunome also marks a conceptual shift in how the immune system’s genetic diversity could be studied in health and diseases, this newsletter will delve into the immunome’s role in cancer prevention and treatment.
The Immunome in Health and Disease
One of the fundamental research into the immunome and diseases was rather recent in a 2019 study from the Israel Institute of Technology and U.S Stanford University. This longitudinal study captured the unique immunome of 135 healthy adults of various ages over nine years, noting that ageing affects 33 types of immune cells. Some of these cell types change in predictable patterns as people age, either stabilising at a certain level or consistently increasing or decreasing. By analysing how these immune cells change over time, the study computed the immune ageing (IMM-AGE) score to measure the immune system’s age. Importantly, applying this score to a larger cohort showed that it independently predicted the diagnosis of cardiovascular diseases.
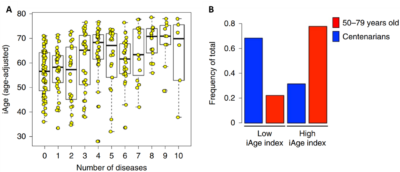
Further extensive studies have demonstrated that the immunome correlates well with ageing, all-cause mortality and the number of chronic diseases (Figure 1A). Conversely, the immunome is also closely associated with health, where centenarians (persons who live to be 100 or older) often exhibit an immunome of lower inflammatory index (Figure 1B). The immunome also outperforms typical biomarkers of systemic inflammation, such as C-reactive protein (CRP) or interleukin-6 (IL-6), in identifying overall health and disease outcomes.
Figure 1. The immunome tracks well with the number of chronic diseases (A) and exceptional longevity (B). A healthy immunome is characterised by a low iAge (inflammatory ageing clock) index. Source: Sayed et al. (2021), Nature Aging.
Regarding cancer, the role of the immunome is particularly complex. Cancer is as much an immune-related disease as it is a malignant (abnormal cell growth) one, with patients exhibiting varying immune responses toward the cancer due to differences in their immunome. Some patients can mount effective anti-tumour immune responses that control or eliminate cancer, while others experience tumour progression facilitated by inflammation or immune evasion mechanisms.
In response, cancer cells employ different strategies to counter the host immune system, leading to an immunological arms race. As a result, even among patients with the same type of cancer, tumours often display distinct behaviours and mutation profiles, a phenomenon known as tumour heterogeneity. Therefore, scientists who study the immunome in cancer view it in two ways: the human and tumour immunome. While human immunome refers to all components of the host immune system, the tumour immunome is more specific to the cancer-immune interactions, i.e., the immunological state of the tumour.
The Tumour Immunome
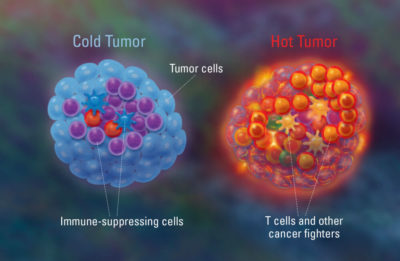
Before the concept of tumour immunome was devised, scientists already had a gist of what it is. Early research efforts into understanding the tumour’s immunological state have distinguished between “cold” and “hot” tumours. Cold tumours are characterised by a lack of immune cell infiltration, making them less responsive to immunotherapies because they appear invisible to the immune system. In contrast, hot tumours have high levels of immune cells within them, indicating an active inflammatory and more chaotic immune response (Figure 2).
Figure 2. An illustration of cold vs. hot tumour. Cold tumours have lower immune activities as the cancer cells are hiding from the immune system. Hot tumours have higher immune activities due to the infiltration of immune cells aimed at attacking the cancer cells, resulting in a pro-inflammatory environment. Source: Freeman (2018), Dana-Farber Cancer Institute.
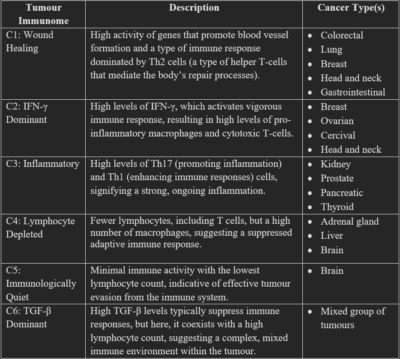
The complexity of the tumour immunome is better illustrated in a pivotal 2018 study, directed by the Institute For Systems Biology of Seattle, U.S. This study analysed the immunome of 10,000 different tumour samples across 33 cancer types using advanced immunogenomic and machine learning techniques. The study then uncovered six distinct tumour subtypes based on their immunological state: wound healing (C1), IFN-γ dominant (C2), inflammatory (C3), lymphocyte depleted (C4), immunologically quiet (C5) and TGF-β dominant (C6). Descriptions of these subtypes and which cancer they commonly associate with are summarised in Table 1.
“In summary, six stable and reproducible immune subtypes were found to encompass nearly all human malignancies,” the study authors concluded. “With our increasing understanding that the tumour immune environment plays an important role in prognosis as well as response to therapy, the definition of the immune subtype of a tumour may play a critical role in predicting disease outcome as opposed to relying solely on features specific to individual cancer types.”
Table 1. The six subtypes of tumour immunome. Source: Thorsson et al. (2018), Immunity.
Abbreviations: C1, cluster 1; IFN-γ, interferon-gamma; TGF-β, Transforming growth factor-beta; Th cells, T-helper cells.
Importantly, the study found that these subtypes can predict patient outcomes. For instance, the C3 subtype had the best prognosis in terms of overall survival, followed by C1, C2 and C5, when adjusted for the cancer type. Subtypes C4 and C6 had the least favourable outcomes, i.e., shortest overall survival, probably due to their more mixed immune signatures. With further research and medical advancements, the study authors hope that immunotherapy will one day be tailored to the specific tumour immunome for optimal personalised therapy.
The Human Immunome in Cancer
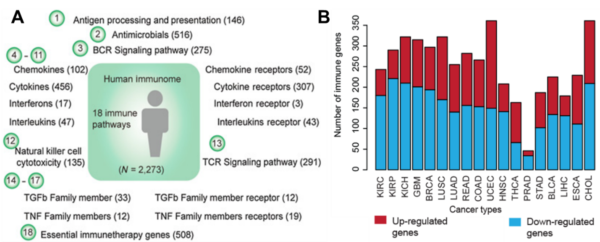
Moving on, research into the human immunome in cancer is relatively nascent than the tumour immunome. In a landmark 2020 study, scientists at the University of Texas categorised the human immunome into 18 immune-related pathways, the activities of which may vary depending on the cancer type (Figure 3). For example, the cytokine receptor pathways (involved in inflammation signalling) were more active and mutated in cancers of the lungs, lymph nodes and skin compared to other cancers. Additionally, most cancers tend to suppress the antigen presentation pathways (involved in threat recognition) to evade the immune system, except for breast and brain cancers. Conversely, patients with breast or brain cancer often exhibit downregulated cytokine receptor pathways, indicating inhibited immune signalling.
Essentially, this study highlights how different cancers can influence the human immunome in multifaceted ways. When combined with earlier findings on the tumour immunome, it is evident that the human and tumour immunome interact with one another, creating a complex network of immunological intricacies that scientists are just beginning to decipher. Decoding this complexity could be the key to designing targeted or personalised immunotherapy for cancer patients.
Figure 3. The human immunome in cancer. (A) The human immunome is categorised into 18 pathways, with the number of genes in each pathway in brackets. (B) The activities of immune-related genes across cancer types. Red for up-regulated genes and blue for down-regulated genes. Source: Li et al. (2020), Cancer Research.
In a 2024 commentary, “Why We Need an Immunome,” Eric Topol, MD, also emphasised the need to better understand the immunome in health and disease, including cancer. Dr Topol is a professor of molecular medicine and founder of Scripps Research Translational Institute, U.S., who is also known as one of the most influential biomedical scientists worldwide.
“With a large body of knowledge indicating the immune system as a (if not the) major determinant for our health and diseases, we desperately need a means for assessing our immune system,” Dr Topol wrote. “It could predict a person’s response to infections, vulnerability to cancer or its spread, no less cardiovascular and neurodegenerative disease susceptibility.”
Importantly, Dr Topol advocates strengthening the immunome with interventions like enhancing gut microbiome health through probiotics and a fibre-rich diet, alongside maintaining a healthy lifestyle with balanced nutrition, regular exercise and adequate sleep. Additional immune support strategies include stress management, vitamin supplements and phytotherapy, i.e., bioactive plant compounds for health and medicinal purposes. Ultimately, it all ties back to the fundamental role of a robust immune system in preventing diseases, maintaining overall health and preserving biological youth.