An Integrative Treatment Approach to Castration-Resistant
and Metastatic Prostate Cancer
by Ben Pfeifer, M.D., Ph.D.
Professor and Director for Research and Development
Aeskulap International, Lucerne, Switzerland
Aeskulap International is combining conventional and complementary oncological treatments to create effective therapies for patients with metastatic and castration-resistant prostate cancer. This integrative approach includes basic complementary treatment measures, such as dietary- and lifestyle changes, specific physical therapy and exercise programs, psycho-oncology measures, hyperthermia- and fever therapies, detoxifying treatments, enzyme therapy and specifically designed medical treatments utilizing various plant derived preparations (phytotherapy).
In Western Europe prostate cancer is the most frequent malignant tumor type in man. Standard hormone-ablation therapies for men with recurring cancer after radical prostatectomy and / or radiation therapy often achieve only a short-term control of tumor growth. Due to clonal selection of hormone independent cancer cells, the so-called castration-resistant (formerly called hormone-refractory) stage of the disease develops in many of these patients. At this stage, therapeutic options are limited to “watch and wait” strategy, second-line hormonal therapies, or eventually chemotherapy which often causes significant side effects without truly extending the patient’s life.
Since each patient with advanced, metastatic, and castration-resistant prostate cancer is different, even if PSA levels and disease history might be similar, Aeskulap International offers tailor-made treatments including a supportive phytotherapy protocol for each individual patient. Depending on specific patient data, a special combination of phytotherapy compounds is chosen from the following product list for treatment: ProstaSol, Curcumin combi, BioBran, IMUPROS, Modified Citrus Pectin, Indole-3-Carbinol, IMUSAN, Convolvulus arvensis extract, and Artemisinin. In situations with very high PSA levels, aggressive tumor histology, and failing second line hormonal treatment (for example: Abiraterone – Zytiga and Enzalutamide – Xtandi), we also prescribe Aeskulap-Sitosterol-Mix, a tailor-made compound that greatly improves the efficacy of our phytotherapy protocol.
The following provides a short summary on the above-mentioned substances used in our treatment protocols; a more detailed information sheet for each substance as well as specific details about our treatment protocol can be requested from our office under:
case-manager@integrative-cancer-care.com
ProstaSol™ contains various plant extracts, all known to support prostate health. The combination of Bio-Curcumin – a highly bio-available form of curcuma, LinumLife – a flax-seed extract rich in lignans, and Resveratrol – a strong antioxidant derived from grape seeds provides anti-inflammatory, anti-oxidative and anti-cancer properties.
Curcumin combi™ is a combination formula with extracts from Turmeric root and Resveratrol from Polygonum cuspidatum. It has powerful anti-oxidative, anti-inflammatory, and anti-tumour activities. Curcuma extracts are utilized worldwide in treatment protocols for patients with cancer. Their beneficial effects have been shown in more than 3000 studies.
IMUPROS™ is an orthomolecular combination formula, containing selenium, zinc and calcium, the vitamins C, D and E, as well as genistein, lycopene and epigallocatechin gallate; it provides the vitamins, minerals, and other vital substances necessary to maintain a healthy function of the prostate gland.
BioBran® is a processed rice bran polysaccharide containing Arabinoxylan as a major component which has a powerful immune modulation effect. This compound causes overall stimulation of the immune system. In particular, it increases the number and function of T-cells, B-cells, and NK-cells. BioBran also enhances apoptosis of cancer cells. The substance is non-toxic, and it is easily absorbed after oral intake. The effectiveness of BioBran has been documented in experimental and clinical studies published in peer-reviewed journals. At Aeskulap Hospital in Switzerland, BioBran is considered an important component of integrative tumour therapy for patients with prostate cancer and cancer in general.
Aeskulap CA-Statin contains Convolvulus arvensis extract with various proteoglycans which exert significant anti-angiogenesis effects reducing the blood supply to primary tumour as well as metastatic lesions. This is helping to slow down cancer growth.
Aeskulap Modified Citrus Pectin is a complex water soluble, indigestible polysaccharide obtained from the peel and pulp of citrus fruits and modified by means of high pH and temperature treatment, to affect numerous rate-limiting steps in cancer metastasis. The anti-adhesive properties of MCP help to reduce the metastatic potential of cancer cells. MCP also exerts powerful apoptotic signals forcing in particular metastatic cancer cells to initiate cell death programs in multiple human malignancies.
Indole-3-Carbinol is a special formula combining plant derived substances from the Indole / Carbinol group with Resveratrol. This combination helps to maintain normal cellular function and cell division, provides protection from free oxygen radicals, and modulates the estrogen metabolism. It is used in the adjuvant treatment of breast-, cervical-, and prostate cancer. It is effective in hormone-sensitive as well as in the hormone independent variants of breast- and prostate cancer.
IMUSAN is an extract mixture of fifteen mostly Chinese herbs with proven efficacy against various cancer cell lines and stimulatory effects on natural killer cells. IMUSAN is boosting natural defense mechanisms of the immune system and it supports proper immune function in the fight against cancer.
Artemisinin is a secondary plant compound extracted from “Artemisia annua”. It is used worldwide for treatment of multi-resistant strains of Malaria. Besides Artemisinin, several flavones and other active biologic substances have been isolated from “Artemisia annua” – all demonstrating significant effects against various cancer cells.
Over the last 15 years, more than 10,000 men have used our phytotherapy protocols for treatment of their prostate cancer. Most of these men had reached the castration- resistant stage of the disease and were metastatic when they entered our treatment protocols. There was clinical benefit from our integrative treatment approach in about 2/3 of all men treated with our phytotherapy protocols. This benefit was measured in reduction of PSA levels of more than 50%, reduction of pain from metastases, and improvement in quality of live as well as prolongation of time to disease progression. Currently, a surprisingly large number of men with initially far progressed metastatic prostate cancer (bone, lung, lymph nodes) in the castration-resistant stage of the disease are alive on our treatment protocol, surviving significantly longer than expected by their primary care physicians.
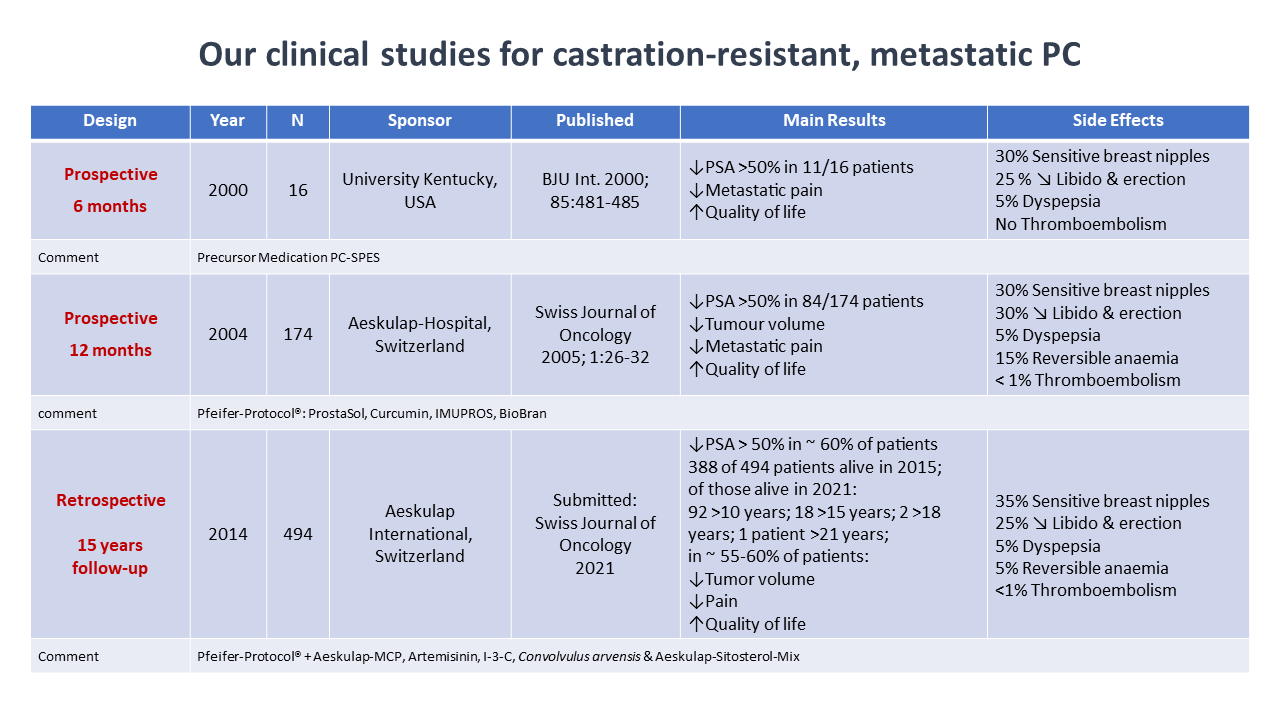
Effectiveness and side effects of our phytotherapy protocols for advanced, metastatic, and castration-resistant prostate cancer were evaluated in two prospective and two retrospective clinical studies with a total of 684 patients evaluated (Figure 1).
Figure 1
In these studies, patients were either treated with a “standard” protocol consisting of BioBran 1g per day, ProstaSol 3 x 3 capsules per day, Curcumin 3 x 1 capsule per day, and IMUPROS at a dose of 2 to 3 tablets per day, or they received a tailor-made treatment protocol including additional phytotherapeutic compounds as needed and adjusting the treatment dosages according to PSA levels and clinical response. Efficacy and toxicity of the respective treatment protocol was assessed by prostate specific antigen (PSA), quality of life parameters and regular blood laboratory analysis for these patients. Effect of the treatment protocol on overall tumor volume was determined by CT scan, bone scan and MRI evaluation, prior and during the observation period, which was 6 and 12 months, respectively, for the prospective studies, and almost 13 years for the retrospective studies.
- Significant PSA declines were observed in two thirds (68%) of the patients on the protocol.
- Reduction of tumor volume was seen in about half of the patients, and 65% of patients reported improvement in their quality of life, in particular a reduction of pain.
- Side effects, such as soreness of breast nipples and dyspepsia were reported in about 35% and 5%, respectively. Thrombotic events and/or pulmonary embolism were recorded in 4 patients.
Clinical benefit from the program lasted on average 4.7 years. To date, 106 of these men have died due to disease progression. The remainder of patients are alive and under continued treatment with our protocols. Remarkably, 28 patients among these survivors live with their metastatic disease now for more than 15 years.
Our experience shows that an integrative treatment approach utilizing a complex basic oncology treatment program in combination with specific phytotherapeutic protocols can significantly improve clinical outcome and quality of life for many cancer patients, even in advanced disease stages.