When cancer patients undergo radiotherapy, the goal is clear: destroy the tumour with radiation. But new research has revealed an unexpected side effect. This life-saving treatment may quietly erode at a small piece of male DNA: the Y chromosome. This phenomenon, known as mosaic loss of chromosome Y (mLOY), has been linked to age-related diseases, such as heart disease, dementia and certain cancers. Now, researchers are asking an unsettling question: could a therapy designed to save lives also be accelerating biological ageing in men?
What is Mosaic Loss of Chromosome Y (mLOY)?
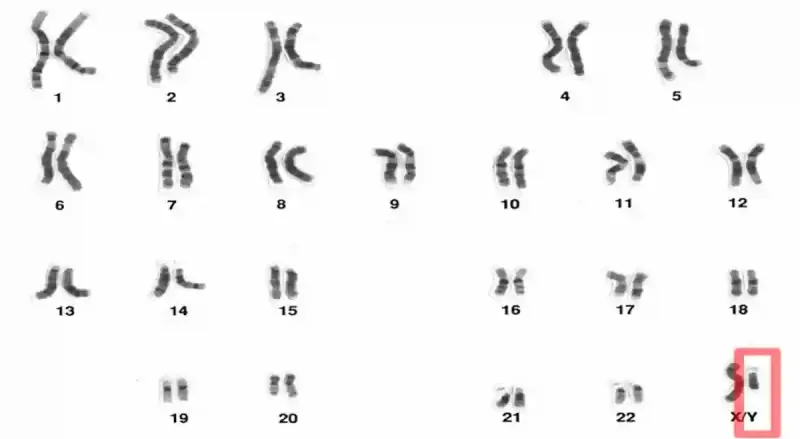
Our cells can pick up genetic changes over time, even when they are not cancerous. Some changes are tiny like swapping out a single letter of DNA, while others are larger, such as losing an entire chromosome. Chromosomes are packages of tightly coiled DNA, and each human cell normally contains 46 chromosomes, arranged in 23 pairs. The first 22 pairs are autosomes (non-sex chromosomes), which are the same in both sexes. Autosomes carry nearly all the genetic instructions for building and running the human body, except for determining biological sex. That task belongs to the 23rd chromosome pair, the sex chromosomes, where females have two X chromosomes (XX) and males have one X and one Y (XY) (Figure 1).
Figure 1. Human chromosomes are arranged in 23 pairs. The first 22 pairs are autosomes, while the 23rd pair is the sex chromosomes. In males, the sex chromosomes are X and Y (XY, highlighted in red); in females, both are X (XX). The Y chromosome is much smaller than the X chromosome and can be lost in some cells with age, a phenomenon called mosaic loss of chromosome Y (mLOY). Source: National Human Genome Research Institute.
In older men, one of the most common large-scale genetic changes is the loss of the Y chromosome in some cells, a phenomenon known as mosaic loss of chromosome Y (mLOY). It is mosaic because only some cells are affected, creating a mix of normal and Y-lacking cells. Fewer than 2% of men under 60 have detectable mLOY in their blood cells, but the occurrence can climb to 40% by age 85. Certain genetic traits and lifestyle exposures can make mLOY appear earlier as well. While lifestyle factors such as air pollution and heavy alcohol use have been linked to higher rates of mLOY, smoking stands out as the strongest risk factor. Smokers are up to four times more likely to exhibit mLOY compared to non-smokers, with heavier tobacco use linked to greater loss. However, this damage is partly reversible after smoking cessation.
Why is the Y chromosome so vulnerable? It is smaller than the X chromosome and carries fewer non-essential genes for cell survival. After all, females do not even have a single Y chromosome in their cells. This means a cell can lose its Y chromosome and still function well enough. As men age, the cell’s machinery for accurately copying and distributing chromosomes also becomes less reliable. This problem is made worse by factors such as smoking, excessive alcohol consumption and chronic inflammation. As a result, the Y chromosome is both easier and less costly to lose than its X counterpart.
Is losing the Y chromosome a problem though? At first, scientists thought mLOY was just part of normal male ageing. However, growing research has linked mLOY to various long-term health problems, including heart disease, dementia and solid cancers. For instance, men with prostate or colorectal cancers had about 10% more blood cells missing the Y chromosome compared to cancer-free men. Large cohort studies from the U.S., the U.K. and Sweden have also found that mLOY is tied to a higher risk of developing and dying from solid cancers.
(Note: solid cancers are tumours that grow in organs or tissues, rather than cancers that start in the blood or immune system, such as leukaemia or lymphoma.)
Radiotherapy Accelerated mLOY, New Study Finds
Interestingly, the link between mLOY and cancer seems stronger at the time of, and after, a cancer diagnosis than before it. In other words, men tend to show more cells lacking the Y chromosome after a cancer diagnosis. This raises the possibility that cancer therapies themselves might speed up mLOY. However, the connection between cancer therapy and mLOY had not been thoroughly investigated until recently.
In a 2025 study, Japanese scientists from Juntendo University, Tokyo, examined whether different cancer therapies were linked to mLOY in men with prostate, lung, colorectal and gastric cancers. They first analysed 348 prostate cancer patients treated at Juntendo University Hospital, using genotyping arrays to scan thousands of sites across the chromosomes. A weaker-than-expected signal from Y-specific genetic markers indicated that some cells were missing the Y chromosome. Patients were then grouped by the therapy they received (i.e., surgery, hormone therapy or radiotherapy), and the prevalence of mLOY was compared.
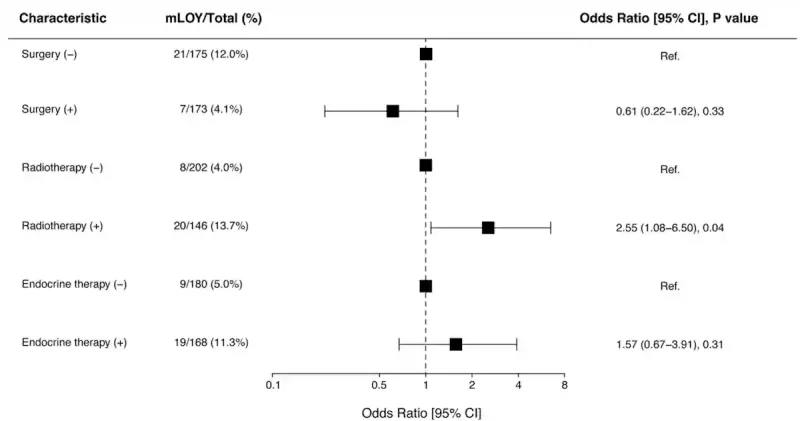
Results revealed that men who had received radiotherapy were about three times more likely to show mLOY (13.7%) compared with those who did not receive radiotherapy (4.0%). In contrast, surgery and hormonal (endocrine) therapy were not significantly associated with mLOY. This finding suggests that mLOY was specific to radiation exposure rather than cancer therapy in general (Figure 2).
Figure 2. In men with prostate cancer, those who had radiotherapy were more than twice as likely to show loss of the Y chromosome in their blood cells (13.7%) compared to those who did not (4.0%). Surgery and hormonal (endocrine) therapy were not linked to a significant increase in Y chromosome loss. Source: Kobayashi et al. (2025), NPJ Ageing.
The researchers proceeded to test whether these findings hold true in a larger and more diverse population with the Biobank Japan dataset. This cohort included over 30,000 male patients diagnosed with prostate, lung, colorectal or gastric cancer. Across all four cancer types, men who had undergone radiotherapy had higher odds of mLOY compared with those who had not. The strongest associations were seen in prostate and lung cancers, where roughly one in three radiotherapy-treated men had mLOY in their blood cells. These associations remained statistically significant even after adjusting for age, smoking and other variables.
The dose and type of radiotherapy also mattered. Higher radiation doses to the pelvic bone marrow correlated with greater odds of mLOY. The study authors suggest that this may reflect radiation-induced DNA damage to stem cells in the bone marrow, which are responsible for producing new blood cells. In line with this, the risk of mLOY was observed with external beam radiotherapy but not for brachytherapy (internal radiotherapy). As brachytherapy places radioactive sources inside the tumour, it minimises radiation spill to the surrounding tissues. As a result, the bone marrow receives less radiation exposure, reducing the likelihood of DNA damage to stem cells and subsequent mLOY. In contrast, external beam radiotherapy delivers radiation through the skin, muscle and bone marrow before reaching the tumour.
(Why not just switch to brachytherapy entirely? While safer for bone marrow, brachytherapy is not suitable for every patient or cancer type. It works best for small, well-defined tumours that are easy to reach. For larger, more irregular tumours in difficult locations, external beam radiotherapy can cover the area more effectively.)
Radiotherapy and mLOY: The Biological Underpinnings
How could radiotherapy, designed to target tumours, end up erasing the Y chromosome from some blood cells? The answer lies in what happens to the bone marrow during radiotherapy. Bone marrow is the body’s blood cell factory, housing stem cells that produce fresh blood cells throughout life. As these stem cells are constantly copying DNA and dividing to keep up with the blood supply needs of the body, their DNA are more vulnerable to radiation damage.
Radiotherapy works by damaging the DNA of rapidly dividing cancer cells, but the radiation beam can also strike healthy cells in its path, including the stem cells in bone marrow that produce blood cells. In fact, haematological (blood-related) toxicity is a well-known side effect of radiotherapy in which bone marrow and blood cell counts drop. When a damaged stem cell attempts to divide, the process of chromosome segregation (ensuring chromosomes are split evenly) can go wrong. If the Y chromosome is misplaced or too severely damaged, it may be lost entirely in the new cell. The finding that higher radiation doses to the bone marrow correlated with a greater risk of mLOY strongly supports this direct-damage mechanism.
“One possible explanation involves the direct irradiation of haematopoietically active bone marrow during treatment,” the study authors wrote to explain how radiotherapy could lead to mLOY. “In cancers such as prostate and lung cancer, radiotherapy often includes anatomical regions rich in active bone marrow – such as the pelvis, ribs, sternum, and thoracic and lumbar spine – which together account for the majority of adult haematopoiesis.”
(Note: Haematopoiesis refers to the process of blood cell production.)
If radiotherapy can accelerate the loss of chromosome Y, it may speed up biological ageing and raise the risk for conditions already linked to mLOY, such as heart disease and dementia. However, the study authors cautioned that more research is needed to clarify the long-term consequences of elevated mLOY after radiotherapy. While radiotherapy is often life-saving, these findings suggest that monitoring mLOY in male cancer patients could help identify those at higher risk of late complications, allowing for more personalised treatment strategies.
“Our findings suggest that radiotherapy may exacerbate genomic instability by disrupting the balance between DNA damage and the DNA repair response, indicating a potential vulnerability in certain cancer patients to DNA damage induced by radiation therapy,” the authors continued. “Identifying such vulnerabilities could inform personalised treatment strategies, potentially mitigating the adverse effects of radiotherapy.”
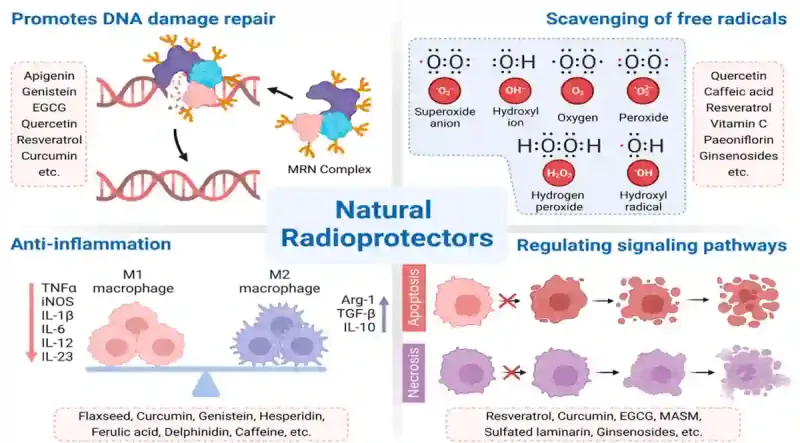
Future research could also explore strategies to protect bone marrow during radiotherapy. Advanced radiotherapy techniques, such as intensity-modulated radiotherapy (IMRT) or proton therapy, can deliver precise doses to the tumour while sparing surrounding bone marrow. Radioprotective drugs like amifostine and palifermin, which can shield healthy cells from DNA damage, show promise as well. Another potential avenue is radioprotective phytotherapy. Certain plant compounds, such as curcumin, resveratrol and green tea catechins, have been found to activate DNA repair enzymes and suppress oxidative stress to facilitate the repair of DNA breaks (Figure 3). However, whether such plant compounds could protect cells from radiation and prevent mLOY remains unproven but worth investigating.
Figure 3. An overview of the radioprotective properties of certain plant compounds. These natural radioprotectors work in several ways: repairing DNA damage, neutralising harmful free radicals, reducing inflammation and regulating cell death pathways. Examples include curcumin (from turmeric), resveratrol (from grapes or berries), epigallocatechin gallate or EGCG (from green tea), quercetin (from onions or apples), genistein (from soy) and ginsenosides (from ginseng), among others. Source: Zhang et al. (2023), Cancers.
Conclusion
The landmark Japanese study offers the strongest evidence yet that radiotherapy, particularly external beam treatment, can accelerate the loss of the Y chromosome in men. While mLOY may seem like an obscure genetic quirk, its relationship to ageing-related diseases suggests it is an important biomarker for long-term health. These findings do not change the fact that radiotherapy remains a cornerstone of cancer therapy, but they do highlight the need to better understand and potentially mitigate its unintended effects on healthy cells, including the blood-forming stem cells of the bone marrow. Protecting these cells could prove critical for preserving long-term health in cancer survivors.