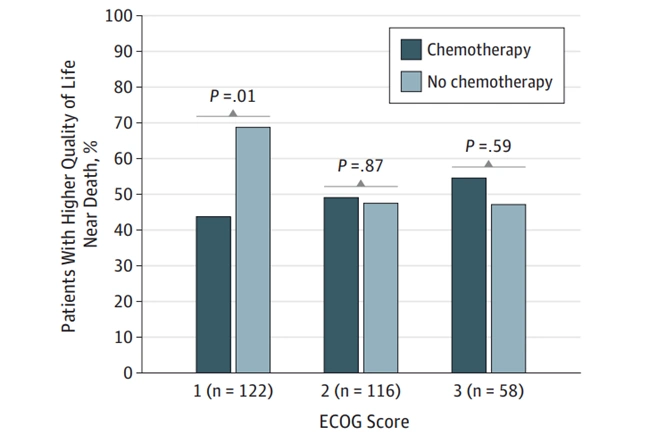Mustard gas was a chemical weapon of mass destruction during World War I, which caused severe blisters, eye damage and life-threatening multi-organ failure upon contact (Figure 1). Its use was so immoral and hideous that countries vowed never to use mustard gas in warfare.
Amidst this devastation, scientists noticed that soldiers exposed to mustard gas had dramatically low white blood cells. This discovery led to efforts in repurposing nitrogen mustard, a derivative of mustard gas, to kill blood cancer cells in leukaemia patients. The first chemotherapy (anti-cancer drug) was invented, marking a monumental paradigm shift in cancer therapy.
However, we should remember that mustard gas is highly toxic. Leukaemia patients treated with nitrogen mustard only achieved temporary relief, with worse systemic chemotoxicity later on. As a result, nitrogen mustard was abandoned by the medical community, and optimism surrounding chemotherapy research disappeared in the scientific community.
Figure 1. ‘Gassed’ art by John Singer Sargent (1460), depicting the aftermath of a mustard gas attack faced by British soldiers. Soldiers had their damaged eyes bandaged and had to hold the shoulder of the man in front as they walked. Source: Imperial War Museum.
The Present State of Chemotherapy
Despite initial setbacks, several scientists remained steadfast in their pursuit of anti-cancer drugs. Eventually, progress in this research field managed to innovate chemotherapy drugs with a more favourable harm-to-benefit ratio. Common types of chemotherapy include alkylating agents, anti-metabolites, and topoisomerase inhibitors, which all share one common mechanism: inhibiting cell replication. Cancer cells replicate much faster than normal cells, making them more susceptible to chemotherapy.
Nonetheless, modern research still questions the utility of chemotherapy. A 6-year longitudinal study showed that chemotherapy did not improve survival rates in patients with terminal cancer. Chemotherapy even worsened the quality of life of patients who had good baseline health status, indicating that cancer patients with less severe symptoms had more to lose with chemotherapy (Figure 2). This is ironic, given that chemotherapy is often administered to patients who are relatively healthy and stand a better chance of tolerating and recovering from chemotoxicity.
Figure 2. Chemotherapy use among patients with good baseline performance status (Eastern Cooperative Oncology Group (ECOG) score of 1) was significantly associated with lower quality of life compared to no chemotherapy. Source: Prigerson et al. (2015), JAMA Oncology.
Chemotherapy still has its advantages, however, as it is highly beneficial for a subset of cancer patients. Patients with blood (leukaemia and lymphoma), testicular and ovarian cancer are mainly cured with chemotherapy. In contrast, certain solid cancers of the pancreas, skin and liver benefit less from chemotherapy. Chemotherapy for pancreatic cancer was only effective in reducing pain and improving survival by less than a month in clinical trials. The pancreas has a unique stroma (connective tissue) enclosing it, which stubbornly blocks drug entry. Chemotherapy also has poor bioavailability to the skin, limiting its effectiveness for melanoma. Likewise, liver cancer is usually incurable with chemotherapy due to the rapid development of chemoresistance.
Additionally, chemotherapy can serve as an adjuvant therapy following radiotherapy or surgery to destroy any cancer cells that might have remained. A large cohort study showed that administering chemotherapy to women who were treated for breast cancer reduced the risk of death and cancer recurrence by 25% and 18%, respectively, over 10 years compared to no adjuvant chemotherapy. Chemotherapy can also function as neoadjuvant therapy to shrink tumour size to the extent that it can be surgically removed, which is a common practice with breast, lung and colon cancers.
Unfortunately, chemotherapy, while targeting cancer cells, inadvertently disrupts the replication of healthy cells as well. As a result, chemotherapy is notorious for causing various side effects, which can be mild, severe, or even life-threatening and debilitating. Immediate toxicities often manifest in the skin, hair, bone marrow, digestive system and kidneys. Over time, vital organs like the heart, lungs and brain can be damaged by chemotherapy. More long-term consequences of chemotherapy include infertility and even secondary cancer, which arises from chemotherapy-induced damage to other tissues. Hence, there is a fine line between saving the patient and causing excessive harm with chemotherapy, a major challenge in cancer care to this day.
Using The Pfeifer Protocol to Reduce Chemotoxicity
What if there is a way to reduce chemotoxicity while retaining its cancer-killing potency? This is where complementary cancer therapy comes into play, which aims to enhance the quality of life of patients undergoing conventional cancer therapy such as chemotherapy. Notably, the Pfeifer Protocol stands out as a complementary therapy, which capitalises on the powerful anti-cancer, antioxidant and anti-inflammatory effects of specific plant compounds.
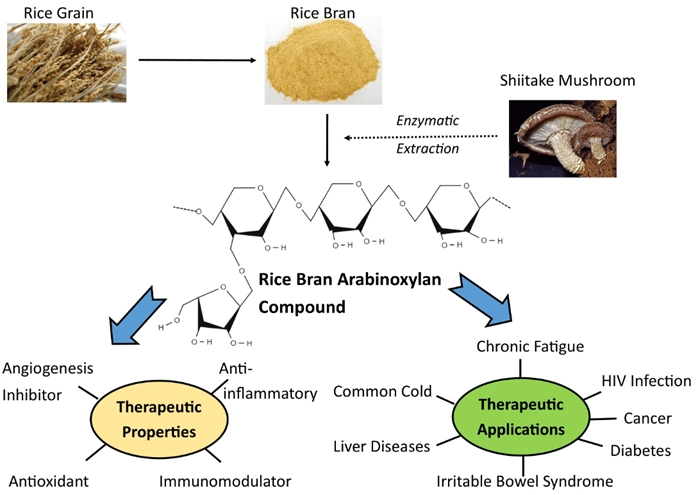
One such compound is BioBran MGN-3, which consists of arabinoxylan extracted from rice bran. Arabinoxylan is a highly potent immunomodulatory compound, capable of reducing inflammation and oxidative stress (Figure 3). A randomised clinical trial showed that consuming BioBran during the week before and after chemotherapy decreased chemotoxicity (i.e., with fewer symptoms of tiredness, anorexia, hair loss and nausea) compared to no BioBran in breast cancer patients. More clinical trials have also reported that BioBran improved immune cell function, quality of life and survival rates in cancer patients on chemotherapy, including breast and prostate cancers.
Figure 3. Rice bran extracted with shiitake mushroom enzyme is known to exhibit antioxidant and anti-inflammatory activities, with wide therapeutic applications, including cancer. Source: Ooi et al. (2021), Molecules.
Aside from BioBran, several components of the Pfeifer Protocol have also been found to reduce the toxic effects of chemotherapy. Here are a few examples:
- Ginsenosides from ginseng: Clinical trials found that ginsenosides enhanced the efficacy of chemotherapy while mitigating its toxicity (e.g., weight loss, gastrointestinal distress, and reduced white blood cell count) in patients with pancreatic, bile duct or lung cancer.
- Shiitake and reishi mushroom extracts: A clinical trial showed that shiitake extract intake improved the quality of life and immunological status of breast cancer patients treated with chemotherapy. Reishi extract also has chemoprotective potential, where it decreased kidney and intestine injuries, vomiting and weight loss in chemotherapy-treated animals.
- Vitamins C and E: A cohort study showed that vitamin C reduced complaints of nausea, anorexia, fatigue, depression, sleep disorders, dizziness and bruising from chemotherapy among breast cancer patients. Vitamin E also exhibits chemoprotective effects in reducing chemotherapy-induced neuropathy (nerve pain) in cancer patients.
The accidental discovery of chemotherapy has experienced highs and lows throughout its clinical journey. Though the efficacy of chemotherapy is marred by its toxicity, innovations like the Pfeifer Protocol bring renewed optimism. By integrating conventional and complementary therapy, we can achieve more effective yet safer cancer care. Instead of solely targeting the cancer with chemotherapy, holistic cancer care should also prioritise enhancing the patient’s overall well-being through complementary approaches.