Background
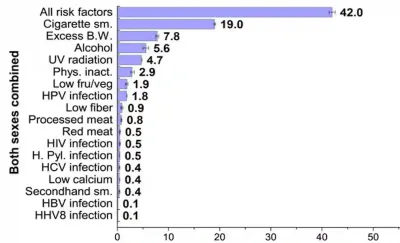
Diseases have various risk factors, some of which are modifiable while others are not. Modifiable risk factors are usually related to lifestyle choices, whereas non-modifiable risk factors include genetics, sex and age, which are beyond our control. About 42% of all cancer cases are estimated to be due to modifiable risk factors, particularly smoking, which is responsible for 19% of cancer cases. Other modifiable risk factors include excess body weight (obesity), alcohol consumption and UV radiation exposure, contributing to about 8%, 6% and 5% of cancer cases, respectively (Figure 1).
As the link between smoking and cancer is well-established, this article will focus on the impact of obesity, the second most important modifiable risk factor, on cancer development. Obesity means having excessive body fat, which is generally measured using the body mass index (BMI) cut-off of over 30. By better understanding the interplay between obesity and cancer, we can reduce cancer risks through informed lifestyle choices and even develop treatments that target biochemical pathways involved in both obesity and cancer. So, let us explore the latest research developments in this field.
Figure 1. Estimated percentage of cancer cases attributable to evaluated risk factors in adults in the United States. Abbreviations/acronyms: B.W. indicates body weight; fru/veg, fruit and vegetable consumption; H. Pyl., Helicobacter pylori; HBV, hepatitis B virus; HCV, hepatitis C virus; HHV8, human herpes virus type 8; HPV, human papillomavirus; Phys. inact., physical inactivity; sm., smoking; UV, ultraviolet radiation. Source: Islami et al. (2018), CA: A Cancer Journal for Clinicians.
Obesity and Cancer: Clinical Implications
The global prevalence of obesity has nearly tripled from 5% in 1975 to 12.5% in 2022, with significant increases observed in most countries. If current trends persist, it is predicted that by 2030, 17% of the global population will be affected by obesity. Consequently, obesity has been regarded as equivalent to a pandemic, albeit with a slower progression and more gradual harmful effects compared to infectious disease pandemics. As a risk factor for cancer, the rising obesity rates would similarly impact cancer incidence. Numerous cancers, including liver, colon, kidney, gallbladder, ovary, breast and prostate cancers, have been associated with obesity.
A 2024 longitudinal study involving over 68,000 post-menopausal women across the U.S. identified both metabolic syndrome and obesity as independent predictors of breast cancer development and mortality over a 20-year follow-up. Metabolic syndrome is a cluster of conditions involving excess body fat, hypertension, and abnormal levels of blood sugar and lipids, which often co-occurs with obesity. Another 2024 longitudinal study from China found that obesity interacts with genetic risk factors in influencing breast cancer risk. Specifically, obesity alone increased the risk by 20%, while a family history of cancer increased it by 60%. When both risk factors were present, the risk of developing breast cancer more than doubled (i.e., 106%). A 2021 meta-analysis of 20 cohort studies further solidifies obesity as a risk factor for breast cancer.
Similarly, obesity has been estimated to raise the risk of prostate cancer by 70%, according to a 2022 meta-analysis that synthesised data from 2.3 million men across 23 studies. A more recent study from Norway found that obese men who gained weight faced a staggering 3-fold increase in prostate cancer risk compared to obese men with stable weight at a 16-year follow-up. Obesity alone also increased the risk of prostate cancer-specific death by 70% in this study. Prior studies have also reported that obese individuals were more likely to develop recurrence of prostate cancer following surgical treatment compared to non-obese individuals.
Further meta-analyses from European and Asian regions have calculated that individuals with obesity were more likely to develop and die from other types of cancer, such as colorectal, gastric, gallbladder, oesophageal, kidney and pancreas cancers. These findings, however, mainly concern those with substantial excess body weight or obesity (BMI of ≥30). In contrast, being merely overweight (BMI of 25-29) may be a protective factor against certain cancers. This paradox may be due to the limitations of BMI as a measure of body fat. Individuals with greater muscle or bone mass also tend to have higher BMI values, potentially confounding the risk assessment.
Obesity and Cancer: Mechanisms
Moving on, let us delve into how excess body fat drives cancer at the cellular level. First, it is well-established that obesity promotes a state of abnormal insulin signalling and chronic inflammation, which create a conducive environment for cancer growth. Elevated insulin levels increase the secretion of insulin-like growth factor 1 (IGF-1), both of which are potent growth-promoting hormones that can exacerbate tumour growth. Chronic inflammation further triggers the release of inflammatory cytokines and reactive oxygen species (ROS), leading to DNA damage and mutations, which are central processes in cancer formation and progression.
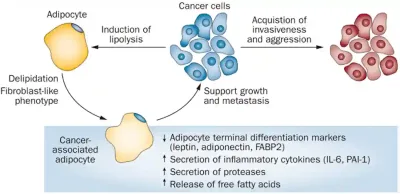
Second, cancer cells can secrete signals that transform neighbouring adipocytes (i.e., fat cells) into cancer-associated adipocytes (CAAs). While normal adipocytes store fat and release energy when needed, CAAs are reprogrammed to release energy-rich fatty acids that directly fuel cancer cell growth, as well as to overproduce growth-promoting hormones and pro-inflammatory cytokines. Moreover, CAAs produce proteases that break down proteins in the surrounding tissue, which helps cancer cells invade and spread to other areas, a process known as metastasis. Interestingly, CAAs also undergo changes to resemble fibroblasts, which are cells involved in tissue repair and structural support. This transformation means CAAs not only aid cancer growth and metastasis but also help build the supportive structure around the tumour (Figure 2).
Figure 2. Crosstalk between cancer-associated adipocytes (CAAs) and cancer cells in facilitating tumour growth and metastasis. Source: Park et al. (2014), Nature Reviews Endocrinology.
Third, emerging research is shedding light on the interplay between adipocytes and cancer stem cells (CSCs). Akin to stem cells, CSCs are a small subpopulation of cancer cells with the ability to self-renew and differentiate into new cancer cells. A major problem is that CSCs are resistant to conventional chemotherapy and radiotherapy, which mainly kill typical cancer cells with high replication rate. As CSCs usually exist in a dormant state, they can evade these treatments and potentially trigger cancer recurrence later. To compound the problem, recent studies show that adipocytes may help cancer cells revert to their stem-like nature, i.e., CSCs.
For instance, a 2023 study found that culturing prostate cancer cells with adipocytes enhanced the expression of CSCs-related molecules, as well as the clonogenicity, survival and invasiveness of prostate cancer cells. Clonogenicity, the capacity of a single cell to grow into a population of cells, is a key feature of CSCs. The co-culture with adipocytes also reduced the sensitivity of prostate cancer cells to chemotherapeutic drugs. “Adipocytes endow prostate cancer cells with stem-like properties,” the study authors concluded, “increasing their tumorigenicity, invasion and chemoresistance.” Similarly, studies in recent years have shown that adipocytes secrete growth factors that enable colon and breast cancer cells to regain their stem cell-like properties.
Therefore, the intricate interactions between adipocytes and cancer cells underscore the significant role of obesity in cancer progression. Understanding such intricacy offers insights into potential therapies that can target these harmful cellular crosstalks, as the next section explores.
Obesity and Cancer: Therapeutic Ventures
The involvement of adipocytes in cancer has been largely overlooked in current medical practice, mainly due to the relatively recent advancements in this research area. Current cancer therapies (e.g., surgery, chemotherapy, and radiotherapy) primarily target the tumour itself without addressing the critical crosstalk between adipocytes and cancer cells, which may help explain why cancer recurrence rates have remained largely unchanged over the past decades.
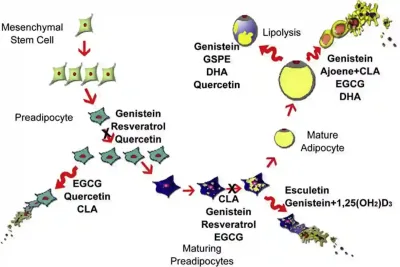
Fortunately, burgeoning research has begun exploring therapeutics that target the adipocyte-related signalling in cancer. For instance, synthetic inhibitors of leptin receptors have exhibited anti-cancer activities in animal models of breast cancer. (Leptin is a growth-promoting hormone that adipocytes secrete.) Other preliminary anti-cancer strategies include targeting the biochemical pathways involved in the synthesis and metabolism of adipocytes using synthetic drugs or natural compounds. Genistein from soy products, curcumin from turmeric, resveratrol from red grapes and epigallocatechin gallate (EGCG) from green tea are some of the plant-derived compounds that can interfere with cellular processes that promote fat accumulation and adipocyte formation (Figure 3). Notably, these plant-derived compounds also form part of our Pfeifer Protocol, designed to complement conventional cancer therapies for enhanced effectivity.
Figure 3. Effects of certain natural compounds on various stages of adipocyte (fat cell) formation. For example, genistein inhibits the proliferation of preadipocytes and reduces lipid accumulation in maturing preadipocytes. It also promotes lipolysis and induces apoptosis in mature adipocytes. EGCG induces apoptosis in both preadipocytes and mature adipocytes and inhibits lipid accumulation in maturing preadipocytes. Quercetin has multiple effects: it inhibits preadipocyte proliferation, induces apoptosis in preadipocytes and stimulates lipolysis in mature adipocytes. Source: Abdel-Sattar et al. (2014), Anti-Obesity Drug Discovery and Development.
Treating obesity can also help combat cancer. Current obesity treatments include bariatric surgery, glucagon-like peptide 1 (GLP-1) medications and lifestyle changes aimed at weight loss. Bariatric surgery involves altering the digestive system, such as reducing the stomach’s size or re-routing the intestines, while GLP-1 medications mimic hormones that regulate appetite. Reduced cancer risk has been observed in obese patients who underwent bariatric surgery compared to those who remained untreated. The use of GLP-1 medications has also been associated with a decreased risk of cancer as an unintended beneficial side effect. A 2007 study by the American Cancer Society found that men who sustained at least 5kg of weight loss for a decade, be it through diet or exercise, had a lower risk of aggressive prostate cancer compared to those with stable weight. A subsequent meta-analysis in 2024 further confirmed the association between weight loss and lower cancer risk.
Overall, the evolving understanding of the relationship between obesity and cancer highlights the need for integrated approaches to both prevention and treatment. As research continues to uncover the mechanisms linking obesity to cancer, it opens the door to innovative therapeutic strategies that could complement existing cancer treatments. Addressing obesity, therefore, is not just a matter of improving overall health but also a crucial step in combating cancer and reducing its recurrence rates. This multifaceted approach will be essential in the ongoing battle against cancer.