Neuropathy is a mild nuisance for some, but it can become an inescapable torment for others. Neuropathic pain can be among the most severe forms of chronic pain known to medicine. When persistent, neuropathy can disrupt daily lives in multiple ways, such as making walking unsteady due to numbness, causing difficulty gripping objects, heightening pain sensitivity to even light touches, interfering with sleep, and leading to fatigue and mental exhaustion. Now, meta-analyses indicate that as many as 25% of chemotherapy patients develop painful and persistent neuropathy. This newsletter explores this critical yet often-overlooked complication in cancer care and, more importantly, what can be done about it.
While chemotherapy is one of the most effective weapons against cancer, it often comes at a cost. Among its most debilitating and underappreciated side effects is chemotherapy-induced peripheral neuropathy (CIPN), where damaged nerves cause pain, numbness, tingling and weakness, typically in the hands and feet. (Peripheral means ‘away from centre,’ such as limbs of the body.) Unlike some chemotherapy’s side effects that improve gradually, neuropathy can persist for months or years and may even be irreversible.
Certain chemotherapy drugs are known to be highly neurotoxic, particularly platinum-based drugs (e.g., cisplatin and oxaliplatin), taxanes (paclitaxel and docetaxel), and vinca alkaloids (vincristine and vinblastine). Their neurotoxic effects arise from unintended interactions with nerve cells or neurons, as their anticancer mechanisms overlap to some degree with essential neuronal functions:
- Platinum-based drugs damage cancer cells by binding to their DNA, creating toxic cross-links that prevent the DNA from repairing itself, which eventually leads to cell death. Unfortunately, they also bind to neuronal DNA, which can directly kill sensory neurons and lead to permanent nerve damage.
- Taxanes stabilise microtubules to block cancer cell division. During cell division, microtubules must assemble and disassemble to separate the DNA, which can be halted by stabilising (locking) the microtubules. However, since microtubules also serve as transport highways inside nerve cells, taxanes disrupt the delivery of nutrients and signals along nerves, leading to neurodegeneration and pain.
- Vinca alkaloids also act on microtubules. Instead of stabilising them, vinca alkaloids prevent their formation to stop cancer cell division. However, microtubules are equally critical for nerve structure and function, so their disruption impairs nerve signalling and weakens neurons, causing tingling, numbness and hypersensitivity to pain.
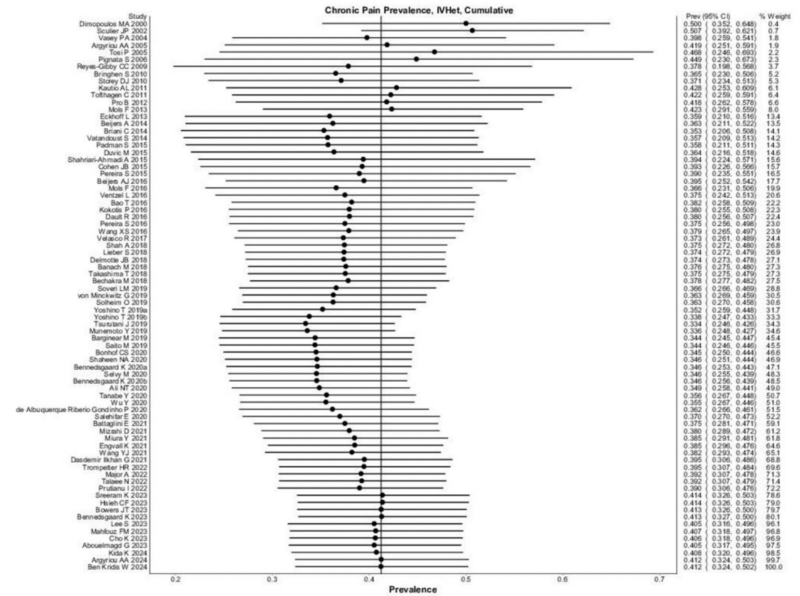
Hence, it is no surprise that chemotherapy patients experience neuropathy more frequently. A 2014 meta-analysis synthesising data from 31 studies found that 70% of patients develop CIPN within the first month of chemotherapy, followed by 60% at three months and 30% at six months. For comparison, neuropathy affects only 2.4% of the general population and 8% of older individuals. However, what remained unclear was how many cases of CIPN are painfully severe. To address this, a 2025 meta-analysis of 77 studies spanning 28 countries estimated that 41% of CIPN patients suffer from severe symptoms lasting at least three months (Figure 1). As expected, platinum-based drugs or taxanes were linked to a higher prevalence of severe CIPN, while insufficient data were available to determine the same for vinca alkaloids.
Figure 1. A meta-analysis of 77 studies to pinpoint the prevalence of painful and persistent chemotherapy-induced peripheral neuropathy (CIPN). The black dots represent individual prevalence rates, which were aggregated to yield an overall pooled prevalence of 41%. The left and right extremes of the dots represent the corresponding 95% confidence intervals. Source: D’Souza et al. (2025), Regional Anaesthesia and Pain Medicine.
Given that 60% of chemotherapy patients develop neuropathy at the three-month mark, and 41% of them experience painful symptoms, it follows that about 25% of all chemotherapy patients suffer from chronic, painful neuropathy (0.41 × 0.60 = 0.246). That means one in four chemotherapy patients endures one of the most debilitating and excruciating clinical conditions. Yet, you may be surprised to learn that no intervention has been formally approved as effective for treating CIPN, leaving many patients with CIPN unsupported.
In 2020, an expert panel from the American Society of Clinical Oncology (ASCO) conducted a systematic review to update guidelines on the management of chemotherapy-induced peripheral neuropathy (CIPN). Systematic review is a type of study that carefully examines all research to summarise the best available evidence on a particular topic. After reviewing hundreds of studies, the ASCO panel still could not recommend any treatment for CIPN, largely due to a lack of definitive evidence from large clinical trials. Many commonly used therapies failed to show consistent, conclusive benefits in CIPN management, including:
- Duloxetine (antidepressant)
- Acetyl-L-carnitine (amino acid)
- Gabapentin (anticonvulsant)
- Scrambler therapy (electrical neuromodulation therapy)
- Exercise therapy
- Acupuncture
- Others: all-trans retinoic acid, amifostine, amitriptyline, calcium magnesium, calmangafodipir, cannabinoids, carbamazepine, diethyldithiocarbamate, glutamate, glutathione, goshajinkigan, metformin, minocycline, N-acetylcysteine, nimodipine, omega 3 fatty acids, org-2766, oxcarbazepine, recombinant human leukaemia inhibitory factor, venlafaxine, vitamin B and vitamin E.
However, some of these approaches have a favourable risk-benefit profile, meaning they pose minimal risk while offering potential benefits. As the ASCO panel noted, “Although proof of benefit has not been provided, data suggestive of benefit support that 3 approaches (scrambler therapy, acupuncture, and exercise) may diminish established CIPN symptoms and appear to be reasonably safe.” Nonetheless, they emphasised the need for further research to clarify the effectiveness of these therapies, acknowledging that while the evidence remains inconclusive, these interventions may still provide relief for some patients.
Given this, the only official ASCO recommendation is that clinicians should carefully assess the risks and benefits of chemotherapy drugs known to cause CIPN, particularly in patients with pre-existing neuropathy or conditions that increase neuropathy risk, such as diabetes or a family history of neuropathy. In patients who develop severe or functionally impairing CIPN, clinicians should consider reducing drug doses, delaying or discontinuing chemotherapy, or switching to alternative chemotherapy drugs that do not cause CIPN.
With limited solutions for chemotherapy-induced peripheral neuropathy (CIPN), the American Society of Clinical Oncology (ASCO) guidelines did acknowledge that, in many cases, there is little choice but to explore low-risk interventions with potential benefits. To this end, phytotherapy (phyto- means plant-based) may be worthwhile to consider. Rooted in decades of integrative or complementary medicine, phytotherapy has been found to enhance the efficacy of conventional therapies, including those used in cancer care.
Some plant compounds can act synergistically with chemotherapy to kill cancer cells, which may help reduce the chemotherapy dosage and lower the risk of nerve damage. For example, curcumin, resveratrol, modified citrus pectin, quercetin and artemisinin have demonstrated the ability to sensitise cancer cells to chemotherapy in laboratory settings. Clinical trials have reported that curcumin, ginseng and epigallocatechin-3-gallate (EGCG, green tea extract) improved clinical outcomes and quality of life in chemotherapy patients, while reducing common chemotherapy side effects such as nausea, pain, fatigue, hair loss and anaemia. Nevertheless, further clinical trials are required to determine whether phytotherapy can safely and effectively reduce chemotherapy dosages and the incidence of CIPN.
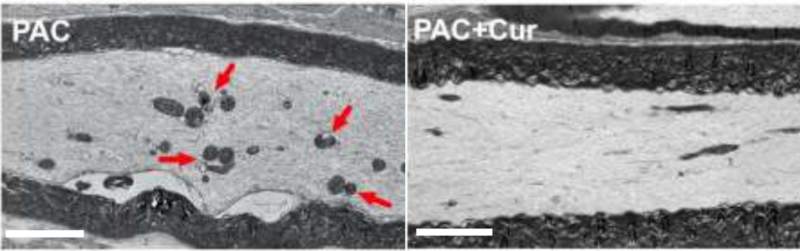
Multiple pre-clinical studies using animal models of CIPN have shown that plant compounds like lycopene, curcumin, EGCG and quercetin could mitigate neuropathic pain. For instance, a 2022 study treated mice with paclitaxel, a chemotherapy drug of the taxane class, to induce peripheral neuropathy. Electron microscopy subsequently confirmed the presence of mitochondrial damage in the sciatic nerve, which connects the leg and foot to the spinal cord. Curcumin administration, however, prevented paclitaxel-induced neuropathy and sciatica nerve damage by acting on the alpha-7 nicotinic acetylcholine receptors to mediate anti-inflammatory and neuroprotective activities (Figure 2).
Figure 2. Electron microscopy of the sciatic nerve of mice with paclitaxel-induced peripheral neuropathy. Damaged mitochondrial structures (red arrows) were seen in the paclitaxel (PAC) group and not in the paclitaxel plus curcumin (Cur) group. Source: Caillaud et al. (2022), Pharmaceutics.
However, clinical evidence remains scarce. Thus far, only one 2025 clinical trial has evaluated the efficacy of a plant compound, namely curcumin, against CIPN using the gold-standard randomised, double-blind, placebo-controlled design. In this trial, 141 leukaemia patients treated with vincristine chemotherapy – a type of vinca alkaloid – were randomly assigned to either the intervention (curcumin) or control (placebo capsule) group. The randomisation ensures that both groups were balanced in terms of age, gender, body mass index, vincristine dosage and cancer severity. The double-blind design means neither the patients nor researchers involved in data collection knew who received curcumin or placebo. Their results showed that curcumin significantly reduced CIPN incidence and severity compared to placebo:
- CIPN occurrence dropped from 70% to 39%.
- Neuropathy symptom scores improved, decreasing from 7.8 to 3.0 points.
- Motor nerve damage in the arm and leg decreased from 19.2% to 10.5%, as measured by electrophysiological nerve conduction studies.
- Myopathy rates fell from 54% to 17%, based on the needle electromyography (EMG) test, which assesses muscle response to nerve stimulation in the arm and leg.
Patients with chemotherapy-induced peripheral neuropathy (CIPN) often describe a burning, stabbing or electric shock-like pain that makes walking difficult, impairs fine motor skills and heightens sensitivity to touch, sometimes to the point where even wearing socks or bedsheets brushing against the skin becomes unbearable. Beyond physical symptoms, CIPN takes a toll on mental well-being and quality of life, making it even harder for cancer survivors to regain a sense of normalcy after treatment. Worse, severe CIPN can force patients to reduce or stop chemotherapy prematurely, which may jeopardise their cancer therapy outcomes.
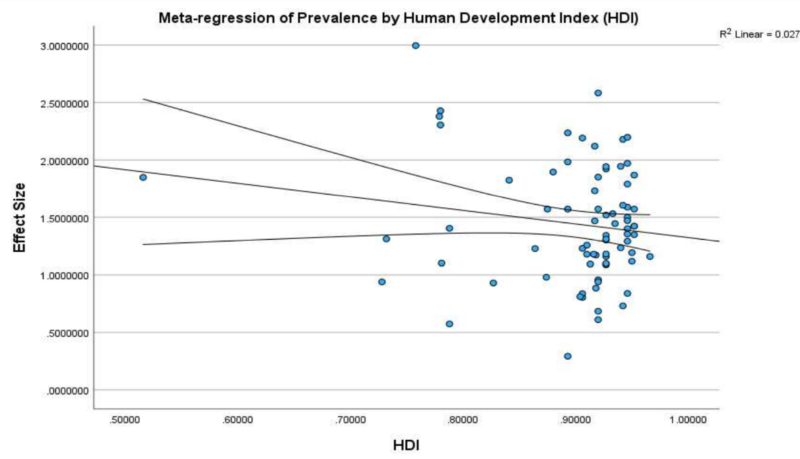
Despite its impact, it is discouraging that no approved treatments exist for CIPN. In fact, the abovementioned 2025 meta-analysis also finds that the prevalence rates of chronic painful CIPN have remained stagnant over time, unaffected by a country’s human development index (HDI) (Figure 3). HDI, which measures life expectancy, education and income levels, typically reflects societal progress, yet CIPN remains an unresolved medical challenge. Urgent action is therefore needed – whether through neuromodulation therapies, novel pharmaceutical drugs or even integrative phytotherapy – to address this pervasive and debilitating condition.
Figure 3. A meta-regression of the prevalence of chemotherapy-induced peripheral neuropathy (CIPN) by human development index (HDI) in the 2025 meta-analysis. A meta-regression analysis is a statistical technique used in meta-analysis to examine how certain variables influence the overall effect size across multiple studies. As shown, the effect size of the prevalence of CIPN remains stable despite increasing HDI. Source: D’Souza et al. (2025), Regional Anaesthesia and Pain Medicine.