Introduction on Radiodermatitis
Emil Grubbe was likely the first physician to use radiation for cancer treatment in Chicago, USA, in 1896. At that time, the danger of repeated exposure to radiation, even at small doses, was not well understood. As a result, Grubbe suffered severe radiation burns and numerous disabilities, necessitating over 90 surgeries to address radiation-induced tumours throughout his body. Similarly, many early radiologists developed cancer from their occupational exposure. Fortunately, this problem stopped when subsequent generations of radiologists began wearing protective equipment to shield against radiation.
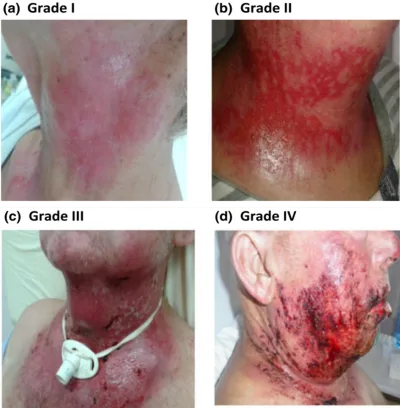
Radiotherapy uses ionising radiation to damage the DNA of cancer cells but inadvertently harm healthy cells as collateral damage. This can cause skin burns or, in more severe cases, secondary cancers due to additional DNA mutations over time. Nonetheless, secondary cancers arising from radiotherapy are rare. In contrast, over 90% of cancer patients undergoing radiotherapy experience radiodermatitis, i.e., radiation-induced skin injury. Although most cases of radiodermatitis involve mild redness (grade 1) to dry peeling (grade 2), a minority may experience severe, moist peeling (grade 3) and even life-threatening tissue death (grade 4) (Figure 1).
The skin is particularly vulnerable to radiation damage. As a continuously renewing organ, the skin undergoes regular cycles of cell shedding and replication to replace the tiny flakes of skin lost through friction. Radiation disturbs this delicate balance of death and regeneration, which can be difficult to recover. While radiodermatitis may resolve over time, it can greatly affect the patient’s quality of life and interfere with the radiotherapy regimen. More concerningly, about one-third of patients experience chronic radiodermatitis for more than 10 years after radiotherapy. Thankfully, new research suggests that the skin microbiome may be involved in mitigating the severity of radiodermatitis. This article delves into the emerging research in this area, highlighting the potential therapeutic implications of the skin microbiome in radiodermatitis.
Figure 1. Grade 1 to 4 radiodermatitis in patients with head and neck cancer. Source: Villavicencio et al. (2001), International Journal of Dermatology.
Your Skin Microbiome Matters
The skin microbiome harbours millions of microbes, mainly bacterial species from the genera Cutibacterium and Staphylococcus. These commensal bacteria form the normal flora of the skin microbiome and play essential roles in warding off foreign, fostering healthy metabolism of skin cells, and interacting with the immune system to prevent diseases. Consequently, imbalances in the skin microbiome have been linked to various skin diseases, including acne, eczema (or atopic dermatitis), chronic wound infections and, more recently, radiodermatitis.
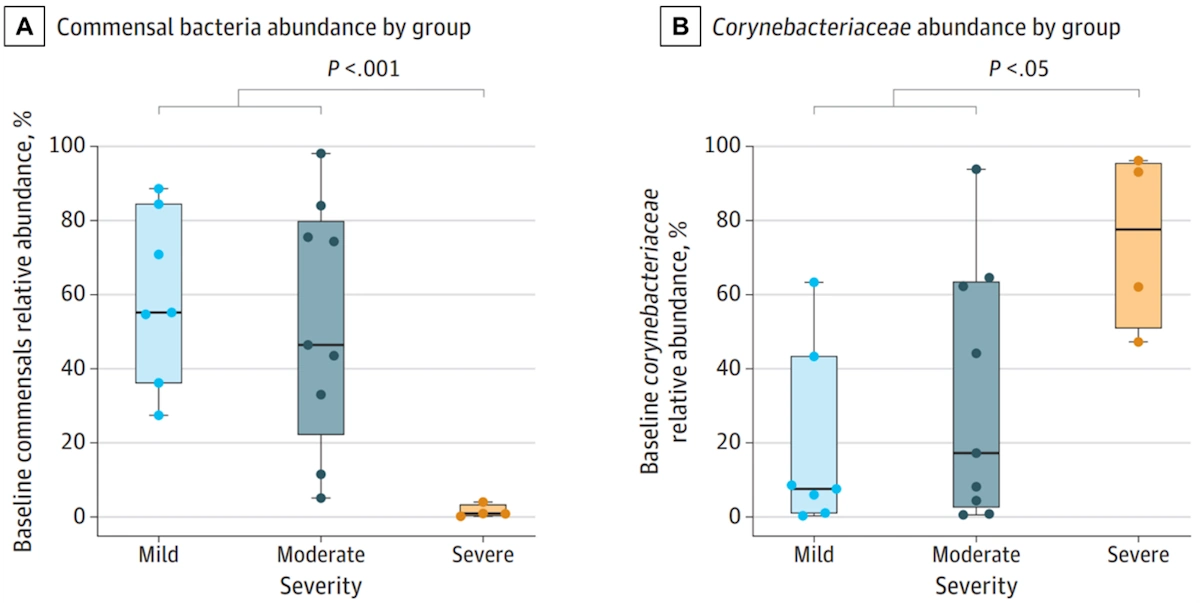
In a 2024 study published in JAMA Oncology, scientists from Germany conducted a longitudinal study to examine alterations in the skin microbiome of breast cancer patients undergoing radiotherapy. Twenty 20 patients were recruited, of which 35%, 45% and 20% developed grade 1, 2 and 3 radiodermatitis, respectively. Through genetic analyses of 360 skin swabs taken before, during and after radiotherapy, the study found that low abundance (<5%) of baseline commensal skin bacteria (Staphylococcus epidermidis, Staphylococcus hominis and Cutibacterium acnes) predicted the development of severe radiodermatitis (Figure 2A). Other factors like body mass index (BMI), breast volume and skin pH were not associated with radiodermatitis severity. Additionally, an overgrowth of non-commensal Corynebacterium species was observed in patients who developed severe radiodermatitis (Figure 2B). Such findings indicate that an imbalanced skin microbiome may worsen the severity of radiodermatitis in breast cancer patients.
Figure 2. Differences in skin microbiome composition between breast cancer patients who developed mild, moderate and severe radiodermatitis after radiotherapy. At baseline, patients who developed severe radiodermatitis had (A) a low (<5%) abundance of commensal skin bacteria but (B) a high abundance of non-commensal Corynebacterium species compared to patients who developed only mild-to-moderate radiodermatitis. Commensal skin bacteria refer to Cutibacterium acnes, Staphylococcus epidermidis, and Staphylococcus hominis species, which form the normal flora of the skin microbiome. Source: Hülpüsch et al. (2024), JAMA Oncology.
As the study authors concluded, “To our knowledge, this study showed for the first time that the pathogenesis of severe radiodermatitis may be mechanistically driven by bacterial overgrowth due to patient-specific predispositions.” Previous studies in 2021-2022 have also reported a shift in the skin microbiome composition of cancer patients who underwent radiotherapy compared to those who did not. This shift was characterised by drastic reductions in both bacterial diversity and the abundance of commensal skin bacteria. These skin microbiome alterations were further correlated with slower healing of radiodermatitis and increased risk of chronic skin ulcers.
Based on research with other skin diseases, overgrowth of non-commensal skin bacteria is known to trigger inflammation and impair the skin barrier. These detrimental effects could exacerbate the DNA damage that radiotherapy delivers to skin cells, resulting in more severe radiodermatitis. Conversely, a healthy skin microbiome marked by a high abundance of commensal bacteria could mitigate inflammation and lessen radiodermatitis severity. Fortunately, we can improve our skin microbiome composition through lifestyle choices, as the next section explores.
Fostering a Healthy Skin Microbiome
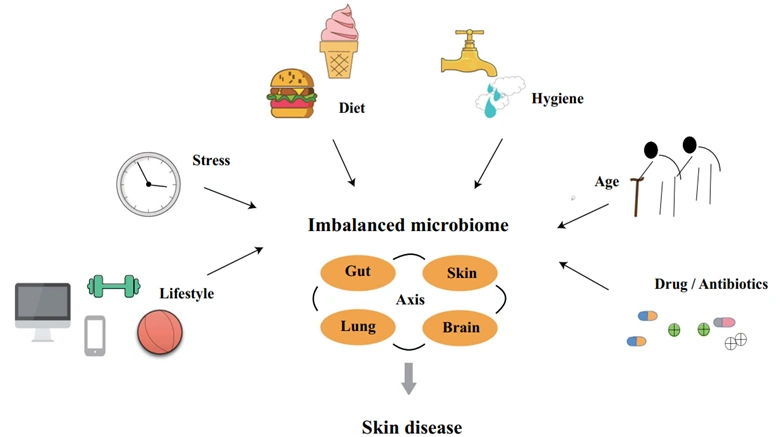
The skin microbiome composition is influenced by various factors, including genetic makeup, environmental exposures, and lifestyle choices (Figure 3). Genetic differences in skin pH and sweat composition can create distinct microenvironments that favour the growth of different microbial communities. Exposure to high levels of pollutants, such as particulate matter, smoke and industrial chemicals, can damage the skin barrier and disrupt the microbial balance of the skin microbiome. These factors, however, are considered non-modifiable because they are difficult, if not impossible, to control.
Conversely, focusing on modifiable lifestyle factors provides a proactive approach to nurturing the skin microbiome. Behaviours involving regular exercise, clean hygiene and healthy diet have been shown to delay skin ageing, prevent skin diseases and facilitate a well-balanced skin microbiome. The role of diet, particularly, has become more crucial owing to the recent understanding of the gut-skin axis. Gastrointestinal and skin disorders tend to co-occur; for example, about 10% of patients with inflammatory bowel disease also have psoriasis, a skin disease that typically affects only 1-2% of the general population. Moreover, strategies aimed at enhancing gut health, such as limiting sugar intake, adopting a Mediterranean diet and consuming probiotics, have been applied as complementary therapies to treat skin diseases in clinical practice.
Figure 3. The microbiome, including the skin, can be affected by various factors, including lifestyle, stress, diet, hygiene, age and antibiotic use. An altered microbiome may consequently influence the development of skin diseases. Source: Yang et al. (2022), Microbial Cell Factories.
A more contemporary approach to targeting the gut-skin axis is phytotherapy, which uses plant-derived compounds to combat diseases. For example, our Pfeifer Protocol offers an individualised blend of diverse plant compounds with potent anti-cancer, anti-inflammatory and antioxidative properties. Some of these compounds have also shown promise in enhancing skin health through the gut-skin axis:
- Lycopene from tomatoes: A 2019 clinical trial reported that lycopene supplementation for one month facilitated the growth of beneficial microbes in the gut microbiome of healthy adults. Such an effect was also correlated with improved parameters related to skin health, such as reduced levels of non-commensal skin bacteria and enhanced lubrication.
- Ginsenoside from ginseng: A 2023 study found that oral ginsenoside enriched propionate-producing bacteria in the gut microbiome. As a potent anti-inflammatory compound, propionate subsequently inhibited inflammatory responses in the gut and skin of a mouse model of atopic dermatitis, reducing the disease severity.
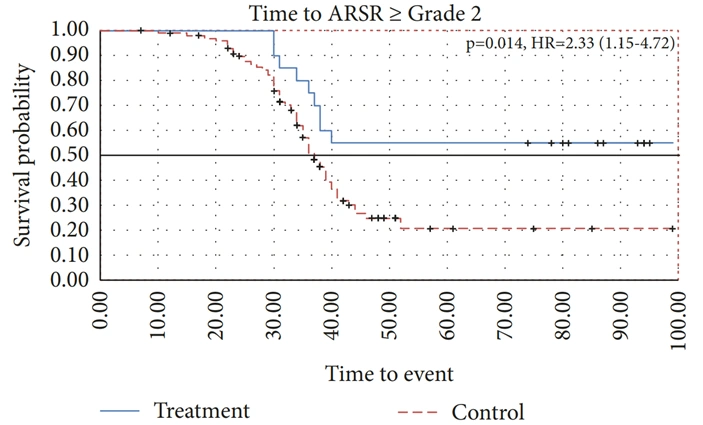
- Epigallocatechin gallate (ECGC) from green tea: A 2019 study showed that ECGC supplementation reversed the skin rashes and altered gut and skin microbiomes caused by prolonged exposure to ultraviolet light in mice. More interestingly, a 2018 pilot clinical trial found that breast cancer patients who applied OM24®, a green tea extract-based skin gel, were more resistant to radiodermatitis than controls (Figure 4). The OM24® skin gel is a patented product of Novelpharm, Switzerland, which uses a unique formulation of ECGC and other bioactive compounds from green tea designed to enhance skin health.
In conclusion, the evolving understanding of the gut-skin axis offers a new perspective on skin health, underscoring the skin microbiome’s influence on skin diseases. This is exemplified with radiodermatitis, where recent research shows that its severity and recovery process partially depend on the skin microbiome. Therefore, it is essential to acknowledge your skin microbiome’s impacts and to engage in practices that support its health and equilibrium.
Figure 4. Time to the development of radiodermatitis or acute radiation-induced skin reaction (ASMR) of grade 2 and above among breast cancer patients treated with OM24 compared to no treatment. OM24 is a patented formulation of skin gel, containing bioactive compounds from green tea. The graph showed that OM24-treated patients had a 2.3-times slower progression to grade 2 or higher radiodermatitis compared to untreated patients, under similar radiotherapy protocols. Source: Näf et al. (2018), International Journal of Breast Cancer.