Understanding the little-known risks (and how to minimize those) of prostate cancer biopsy
Shin Jie Yong and Ben Pfeifer
1. Hypothesis
Progress in medical science has led to a massive advancement in cancer diagnostic and treatment over the last decades. While cancer mortality rates have been decreasing over the years, sustaining a good quality of life remains a challenge. For prostate cancer patients, treatment side effects, such as urinary incontinence, erectile dysfunction, bowel dysfunction, and psychological issues, are common and often impact the patients’ life quality. In addition, the cancer recurrence rate remains high for prostate cancer patients, with almost 50% of the men facing an increasing PSA (prostate-specific antigen; protein associated with prostate cancer in the blood) after initial treatment with curative intent. Why? Prostate cancer diagnostic and treatment have become so much better over the years. Where then could be the problem?
We believe that prostate biopsy – the diagnostic procedure used for cancer diagnosis – could be the culprit. Biopsies have remained in medical practice since the 1960s to confirm a prostate cancer diagnosis. While a precise diagnosis is the starting point to tackle cancer, the associated risks must also be examined with a critical eye. Evidence shows that biopsies can dislodge cancer cells from the primary tumour along the needle biopsy track, or into the lymph- and bloodstream. What does that mean for cancer patients in general, and prostate cancer patients in particular? Could it possibly explain the mismatch between advances in cancer treatments and the stagnant and relatively high recurrence rates that have not improved significantly over the last six decades?
2. An Overview of Prostate Cancer
Between the bladder and penis lies the prostate, a walnut-sized gland that nourishes and lubricates the sperm. As prostate cells grow and divide to maintain the gland’s health and functions, prostate cells have to copy their DNA. But this is not an error-free task, and mutations (incorrect DNA code) are bound to occur with time. Some mutations do nothing; other mutations enable excessive cell growth – taking up space, invading other organs, and becoming cancerous. As ageing leads to accumulation of mutations over time, prostate cancer usually targets the older population – roughly 60% of men by age 65 and 80% of men by age 80, but only 0.5% of men younger than 45 years. Globally, prostate cancer ranks second as the most common cancer in men and fifth as the leading cause of death.
Tackling prostate cancer starts with a proper diagnosis. Only then can doctors make informed decisions and propose feasible treatment options for an individual patient, such as surgical removal, radiation therapy, anti-hormonal therapies, or chemotherapy.
In diagnosis, a screening is done first, which is usually the prostate-specific antigen (PSA) test, where a doctor draws blood to check if a certain prostate protein exceeds an average level of 4 ng/ml. If screening suggests possible cancer, more tests are performed to determine the cancer stage and grade. These include imaging techniques (e.g., magnetic resonance imaging (MRI) or ultrasound examination) and biopsy (i.e., minor surgical procedure to collect a sample of cells from the tumour).
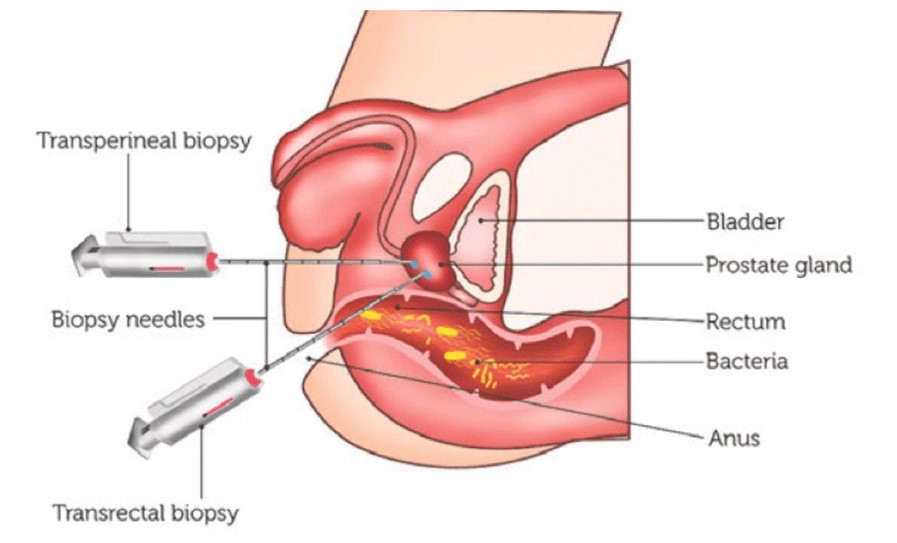
In a prostate biopsy, a thin hollow needle is inserted through the perineal skin (the area between scrotum and anus) or rectum to collect tissue samples from the prostate (Figure 1). An ultrasound or MRI guide may also be used to visualize the needle so that representative tissue sample can be collected. Samples are then analyzed for any cancerous cells. Biopsy is the only definitive method to confirm a prostate cancer diagnosis today. Ultimately, the best way to decipher the tumours’ or cancer cells’ characteristics is to study the cancer tissue directly.
3. The Risk of Biopsy Dislodging Cancer Cells from the Tumor
A 2014 research review of Shyamala Karnam, an associate professor of dentistry, stated that tumours are unstable entities that grow abnormally and invade other tissues. Since they are unstable, they do not hold themselves in place properly, like other healthy cells. Disturbing tumours with needles, or other mechanical stress, would make them even more unstable. In this process, some cancer cells may break free or dislodge from the primary tumour into elsewhere. “Tumor cells are easier to dislodge due to lower cell-to-cell adhesion,” Prof. Karnam et al. wrote. “This theory with the possibility of seeding of tumour cells is supported by several case studies that have shown that after diagnostic biopsy of a tumour, many patients developed cancer at multiple sites and showed the presence of circulating cancer cells in the bloodstream on examination.”
Note that this review refers to cancers in general. Indeed, biopsy-induced dislodging of cancer cells from tumours has happened with many forms of cancers, including breast, liver, stomach, mouth, parotid, lung, brain, kidney, eye, bone, prostate, and probably most, if not all, other cancers. More surprisingly, even the first American textbook on cancer treatments in 1940 did warn about the risks of biopsies: “there is some doubt as to the harmlessness of needling such tumors. It may not be a wholly innocuous procedure.” But the importance of this risk seems to have been diluted over the subsequent textbook versions.
Focusing on prostate cancer, when guidelines for the transperineal prostate biopsy (i.e., needle through the perineum) were published in 1952, the authors raised a theoretical concern that “it is possible to implant cancer cells in the tract of the needle resulting in carcinoma in the perineum.” Just a year later, researchers reported the first case of tumour seeding in the perineum, along the biopsy needle track. In the following years, more and more such cases of perineal tumour seeding appeared in academic journals.
While these are individual case reports, a proper cohort study in 1989 found that perineal tumour seeding occurred in 1% (5 out of 502) of prostate cancer patients. A later study in 1991 showed that tumour seeding could occur after transrectal prostate biopsy (i.e., needle through the rectum) as well, although rarer than transperineal. This study noted a 2% (7 out of 350) total prevalence of tumour seeding following prostate biopsies (transperineal/transrectal).
A few years afterwards, scientists realized that tumour seeding could also occur in the blood circulation after prostate cancer biopsies. In this case, the better phrase should be the dispersion of tumour cells into the bloodstream, creating circulating tumour cells (CTCs), which is many times more common than tumour seeding along the needle track. A few noteworthy studies on this matter are as follows:
This 2014 study is particularly interesting as the researchers looked at cell fragments, measured as epithelial cellular material, rather than solely CTCs. The high prevalence of over 80% means that some cells (cancerous or not) will get inadvertently dislodged from the prostate tumour following a biopsy. As mentioned earlier, “tumour cells are easier to dislodge due to lower cell-to-cell adhesion.” Thus, it is not surprising that biopsy, which pierces the prostate tissue with a high speed and force, would dislodge some cell fragments (including CTCs) into the bloodstream.
As the 2014 study authors stated, “The spring-loaded 18 G needle penetrates the prostate tissue with an average speed of approximately 3–8 m/s, thus causing significant risk for local trauma releasing epithelial cells or cell material into surrounding tissue, lymph, or blood vessels.”
Remember PSA? It is a type of prostate protein that serves as a biomarker for early prostate cancer. Apparently, dozens of studies have reported a rise in blood levels of PSA positive prostate cells following biopsy in about 30-70% of prostate cancer patients, as compiled in a 1998 research review by Eva Correy, a professor of urology. This number also ranged from 0-100%, depending on the technology’s sensitivity. These findings, one of the experimental study stated, “suggest that prostate biopsy might scatter prostate cells in the bloodstream especially in cases with high [PSA] and, thus, might contribute to tumour spreading in the cases of prostate cancer.”
All in all, sufficient evidence shows that biopsies could spread cancer cells into the needle track along the perineum or rectum, or into the bloodstream. As follows, biopsies could increase blood levels of prostate cells (i.e., PSA positive cells or epithelial cellular fragments) and CTCs that may pose adverse health consequences. The latter is particularly concerning as CTCs are one of the primary mechanisms by which cancer spreads and metastasizes to other organs.
4. Dislodged Cancer Cells: Can They Affect Health Outcomes?
Sure, poking through a tumour with a sharp biopsy needle can dislodge cancer cells from it. But what does that mean for a cancer patient? Will dislodged CTCs have an impact on treatment outcomes? Where do those cells go, and what happens to them? Can those cells start new cancer colonies (metastases) elsewhere in the body?
“The clinical significance of [prostate tumour] seeding has not been clearly defined, and there are no guidelines for its prevention or management,” stated another 2014 review of Dimitrios Volanis, MD, PhD, a consultant urologist at the Royal Free London NHS Foundation Trust. “Clinicians are, therefore, often unable to provide evidence-based counselling about the ‘real’ risk of seeding and its impact on prognosis. Indeed, tumour seeding is not mentioned or cited as a risk of biopsy in the European Association of Urology (EAU), American Urology Association (AUA), or UK National Institute for Health and Care Excellence (NICE) guidelines.”
While health authorities have not provided definitive answers on what might happen after prostate cancer biopsy, there are at least five plausible scenarios based on the existing literature:
It is only in a 2020 study that researchers first tracked a group of prostate cancer patients, of whom 29% (i.e., 22 out of 75) had increased CTCs after biopsy, for the next 3.5 years. Results found that 45% (10 out of 22) of those with increased CTCs experienced cancer progression. This number was only 15% (8 out of 52) in prostate cancer patients without CTCs. Importantly, results remain significant even after adjusting for potential confounding variables, such as age, cancer severity, and therapy received. “Because of the number of CTCs before biopsy alone was not correlated with progression of disease, these data supported that the additional mechanical release of prostate cells into the circulation of participants influenced prognosis,” the study authors wrote. This study “should ring a bell for the community, as it provides important evidence that traumatic biopsies are not neutral on disease progression,” cautioned Nicola Aceto, a professor of oncology.
While the 2020 study examined cancer progression, the research on biopsy and recurrence in prostate cancer is limited. To date, there are only case reports. Three cases of cancer recurrences in the bladder wall and perineum struck prostate cancer patients a few years after brachytherapy. This treatment involves needle insertion into the tumour to deliver radiation to kill cancer cells. One incident of tumour seeding into the rectal wall has also been reported recently following a transrectal prostate biopsy. Notably, there is one published case of perineal cancer recurrence 14 years after a transperineal biopsy in a prostate cancer patient. Thorough analyses of the patient’s blood and cancer profiles, the authors wrote, “suggests that the patient would have remained free of disease if the perineal needle tract seeding had not occurred.” However, case studies are just single incidences, which should not be generalized to a broader population.
Since research on biopsy and prostate cancer recurrence is scarce, we can infer insights from other cancers. A 2020 paper analyzed hospital data from 1,047 patients with non-small cell lung cancer, of which 191 patients (18%) faced cancer recurrence after treatment. The researchers found that 20% of this group of 191 patients underwent an imaging-guided biopsy, whereas only 3% in the control (no cancer recurrence) group had the biopsy. That is nearly a seven-times increased risk of cancer recurrence in the biopsy group. Notably, results were adjusted for possible confounding variables, such as age, sex, treatment, and cancer severity. A similar situation applies to colon, breast, and bone cancers, for which biopsies were strongly suspected as the culprit for recurrence.
However, biopsies may be safer for other types of cancers. One 2015 study looked at clinical data of 2,034 patients with pancreatic cancer, of which 498 (24%) underwent ultrasound-guided needle biopsy. After adjusting for con-founders, results revealed lower deaths from pancreatic cancer in the biopsy than control (no biopsy) group – i.e., 50% versus 64% deaths – in a follow-up period of about 21 months. Similarly, in skin cancer, needle biopsies are not associated with any risks of cancer recurrence, concluded a 2016 research review.
All in all, whether biopsy would affect patients’ health outcomes depends on the type of cancer. In prostate cancer, at least we cannot deny that dislodged cancer cells from the tumour into the needle track or bloodstream can happen following biopsy. While tumour seeding into the needle track is rare at 1-2%, biopsy-induced increase in CTCs is much more common, affecting 20-30% of prostate cancer patients. An even more common complication of biopsy is the dislodging of prostate cells (i.e., PSA positive prostate cells or epithelial cell fragments) into the bloodstream, reaching 100% occurrence following biopsy.
As CTCs (and prostate cells) may contribute to cancer progression and recurrence, it could explain why about half of prostate cancer patients suffer cancer recurrence years after surgery and require additional therapies (see section 1: Hypothesis).
5. Minimizing Potential Risks of Biopsies
As long as biopsies are required for cancer diagnosis, risks of tumour seeding into the needle track or bloodstream are inevitable. If we cannot prevent cancer cell dislodging from the tumour following biopsies, what can we do to minimize the possible health risks associated with the dislodging process?
For one, keeping the immune system in top shape, especially during the period before and after biopsies, may help. The immune system is responsible for attacking anything that it determines as foreign and harmful, such as pathogens and cancer cells. This is also why pathogens and cancers will always evolve mechanisms to suppress or evade the immune system or otherwise risk perishing. As follows, a vigilant immune system around the time a biopsy should theoretically kill any cancer cells that might be dislodged from the tumour.
To further err on the safe side, plant extracts have been recommended in the scientific and medical communities. Presently, a few plant extracts are used as an adjunct treatment to reduce the odds and severity of treatment side effects in cancer patients. This efficacy may be attributed to the anti-cancer and immune-modulatory properties of plant extracts. For prostate cancer, a 2019 review summarized several plant extracts that have shown promising results in phase I/II clinical trials in improving the quality of life, disease biomarkers (e.g., PSA), and survival rates of prostate cancer patients.
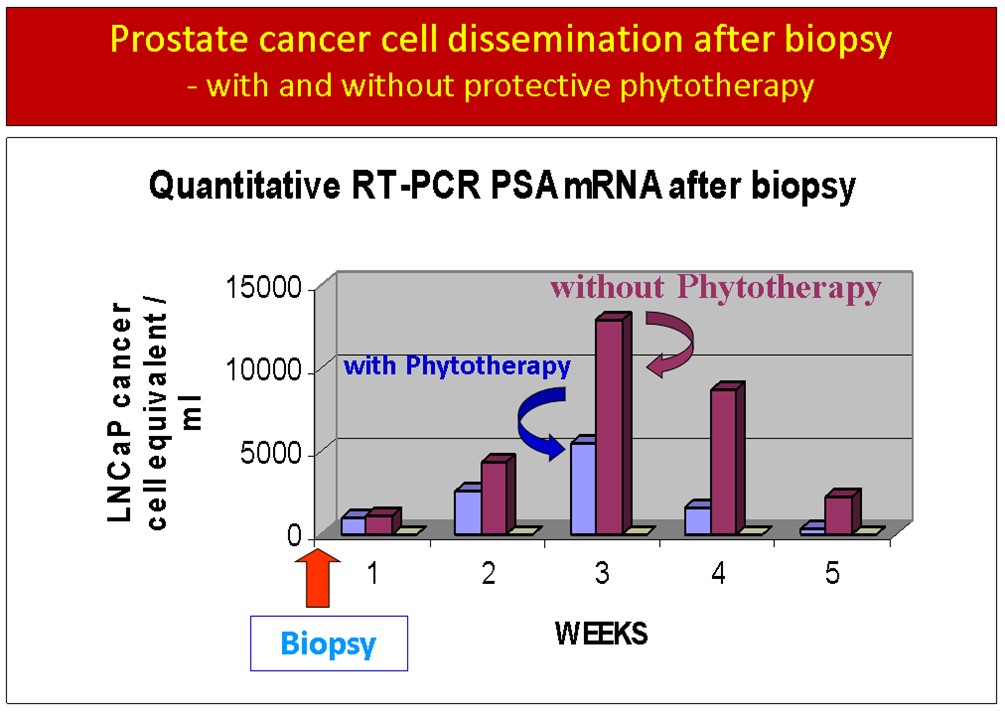
Cell Dissemination Figure 2
Dissemination of PSA positive cells into the peripheral blood of patients after prostate biopsy. Two patient groups were followed with serial blood tests for 5 weeks after biopsy: One group received protective phytotherapy, the other group did not. Note that the treatment group had less than ½ of PSA positive cells in the peripheral blood at week 3 and less than ¼ of PSA positive cells at week 4.
To this end, Ben Pfeifer, MD, PhD, professor, and director of research at Aeskulap International, Switzerland has capitalized on the anti-cancer and immune-modulatory effects of plant extracts to deal with dislodged cancer or prostate cells. “Over [a] decade, we have worked on a protocol to help eliminate those spilled cells as quickly as possible from the blood circulation of our patients after prostate biopsy”, he told me in an email. “Since these cells must either die spontaneously or be killed by the patient’s immune system, our protocol involves natural immune-stimulating remedies, such as BioBran and IMUSAN, and combined those with the anti-inflammatory curcumin, and ProstaSol for prostate cancer cell killing.” An overview of Pfeifer’s protocol for prostate cancer biopsy protection is pointed below, which should be taken ten days before biopsy until day-30 post-biopsy.
- ProstaSol: 3×2 tablets per day
- IMUSAN: 3×1 capsule per day
- BioBran: 3×1 Sachet per day
- Curcumin combi: 3×1 capsule per day
- Aeskulap-MCP: 3×2 capsules per day
The formulations of these products and their respective clinical studies on the health outcomes of prostate cancer patients are detailed elsewhere, which is too lengthy to cover in this article. The main issue herein is if these plant extracts are effective in preventing biopsy complications? Yes, they can. For example, in the preliminary data shown here below (Figure 2), phytotherapy utilizing these plant extracts has effectively blunted the spike in PSA positive prostate cells following biopsy in cancer patients. By week five, such prostate cells have disappeared in the phytotherapy group but remain elevated in the control group.
6. The Future of Biopsies
Presently, the best way to decode the tumour’s characteristics is by sampling it directly via biopsies. Discarding biopsies entirely would not be wise or possible at this time. Hence, current research has considered the potential of liquid biopsies, in which CTCs in the blood are sampled with a simple blood withdrawal, instead of cells in the tumour. Liquid biopsies are, thus, not very invasive and come with minimal risks – just like routine blood sampling.
Akin to biopsies, there have been many developments of liquid biopsy tests based on CTCs, each with their own set of advantages and limitations. While CTC-based liquid biopsies are theoretically free of risks of cancer cells dislodging from the tumour, they still require improvements in accuracy, and are not useable for cancer staging and grading. At this point, based on a 2020 research review, liquid biopsies are approved by the U.S. Food and Drug Administration (FDA) for monitoring the progression of breast, colon, and prostate cancers only; for instance, in predicting probabilities of therapy success or patient survival.
CTCs may also be used as an initial screening to determine if further biopsy is necessary. In a 2011 study, for example, CTCs screening identified 86% of prostate cancer patients (i.e., confirmed via biopsy) compared to 28.6% accuracy with PSA screening. Perhaps only in the future, liquid biopsies would achieve the feat of being as accurate as direct biopsies in cancer diagnoses.
7. Key Points
Biopsies have been linked to tumour recurrence for several cancers, including prostate cancer. The biopsy needle cuts through tissue and can dislodge cancer cells from the tumour along the needle track. Prior research has documented cases of cancer cell seeding after prostate biopsy after perineal and transrectal approach. However, this is a rare complication of prostate biopsies, and modern medicine has chosen to ignore this possible problem.
Another much more common risk of biopsy concerns the health risks of CTCs. Dislodged cancer cells may also find their way into the lymphatic system and bloodstream, entering those through severed veins and lymphatic vessels cut open by the biopsy needle. Whether dislodged cancer cells can survive in the lymphatic system or blood circulation and start new cancer colonies (metastases) is unclear today, but very likely. How else could we explain that the tumour recurrence rate after radical prostatectomy has remained high (~ 45%) over the last decades, despite major improvements in prostate cancer diagnostic and surgical technique? Could it be that the biopsy needed for treatment is the reason for treatment failure in those patients? We feel that this is a very legitimate question that needs a clear answer, quickly.
But it is important to note that such risks are largely outweighed by the benefits of proper diagnosis biopsies offer. Regardless, it is never a wrong move to take safety measures. One precaution is to strengthen the immune system with healthy lifestyle habits, which is especially critical during the week before and after biopsies. Another approach is to supplement with immune-modulatory, anti-cancer plant extracts, as proposed in the Pfeifer Protocol™. These health interventions are perhaps the best we can do to lower the odds of dislodged cancer cells harming our health. This is at least until biopsy methods can transition to liquid biopsies, where there is no longer any risk of cancer cells escaping the tumour.
Anyhow, the overarching aim of this article is not to encourage avoiding biopsies – as they are still needed for proper cancer diagnosis – but to minimize any risks associated with it.