Understanding and Preventing Secondary Cancer after Chemotherapy and Radiotherapy
Introduction
Once considered a death sentence, cancer survival rates have increased dramatically due to decades of medical advancements. While this remarkable progress is worth celebrating, it also brings to light a new challenge: secondary cancer, the development of a new cancer after the primary cancer. The incidence of secondary cancer has risen from <10% before 1980 to a staggering 20% of all cancer diagnoses after 2000. Despite two decades of improved cancer care, the incidence of secondary cancer has stayed persistently high at about 20%.
Secondary cancer often arises from the spread of the initial cancer. However, it may also manifest as a late complication of the therapies used to treat the primary cancer. Therapies such as radiotherapy and chemotherapy are toxic to both cancer and healthy cells. The resulting DNA damage in healthy cells can lead to mutations that elevate the risk of secondary cancer.
This Post aims to explore therapy-related secondary cancer and proactive strategies to reduce its risk. By understanding the triggers and preventive measures, we can better equip ourselves to address the challenges posed by modern cancer therapy.
Radiotherapy-related Secondary Cancer
The fact that excessive radiation causes cancer was first exemplified during the aftermath of the atomic bombing in Japan in 1945. A 50-year longitudinal study revealed a 47% increased risk of cancer per sievert (a unit of radiation exposure) among those who resided within 10 km of the bomb’s hypocentre (ground zero). Afterwards, convincing evidence surfaced that early radiologists developed cancer due to frequent exposure to radiation throughout their careers. Fortunately, radiologists who began to wear protective equipment no longer face an increased risk of cancer compared to the older generation of unaware radiologists.
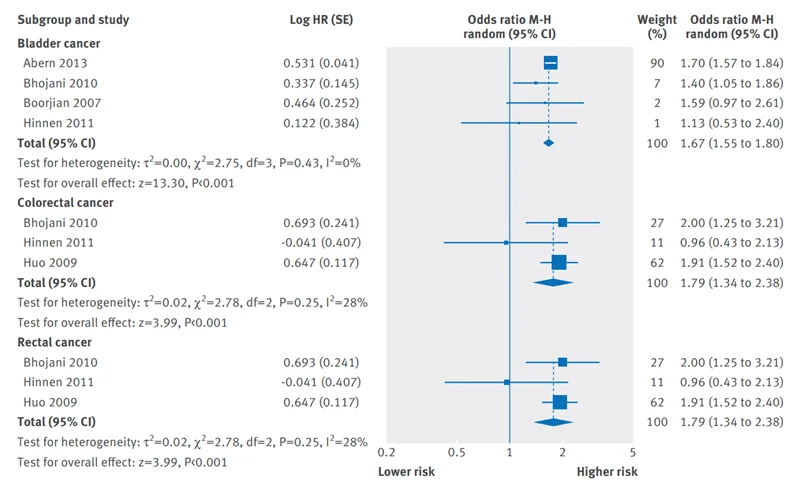
However, cancer patients who had radiotherapy also encounter an elevated risk of cancer, but it may occur years or even decades later. For instance, an early study found that the median period between pelvic radiation and secondary bladder cancer diagnosis was 30 and 16.5 years following low- and high-dose radiotherapy, respectively. Further studies agree that the latency period between radiotherapy and radiation-induced secondary cancer is at least five years. Moreover, a meta-analysis reported that radiotherapy-treated prostate cancer patients had a 67%, 79% and 79% increased risk of secondary bladder-, colorectal- and rectal cancers compared to no radiotherapy (Figure 1). Radiotherapy was also identified as the leading risk factor of secondary brain and blood cancer in cases of childhood cancer.
Figure 1. A meta-analysis showing the risk of secondary cancer among prostate cancer patients after radiotherapy compared to no radiotherapy. For bladder, colorectal and rectal secondary cancer, the meta-analysed results (represented by blue diamonds) lean towards higher risk with statistically significant differences. Source: Wallis et al. (2016), British Medical Journal.
Lifestyle factors may also affect the risk of radiotherapy-induced secondary cancer. One study showed that breast cancer patients who underwent radiotherapy were thrice as likely to develop lung cancer after a decade, with the risk multiplying by 10-fold if the patient is also a smoker. Similarly, smoking doubled the risk of secondary bladder cancer in prostate cancer patients treated with radiotherapy. Cancer patients are, therefore, frequently advised to cease smoking.
Patients receiving radiotherapy are exposed to varying radiation doses in surrounding tissues due to scatter. As cancer risk escalates with higher radiation doses, tissues closest to or within the radiation-treated areas are particularly vulnerable to secondary cancer due to greater scatter doses. As a result, radiotherapy is deemed responsible for about 8% of all secondary cancer diagnoses, according to a large longitudinal study.
Chemotherapy-related Secondary Cancer
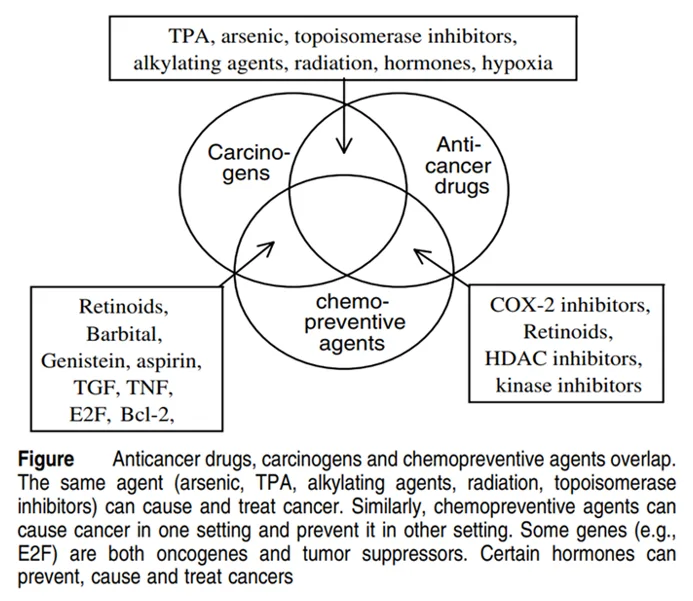
In theory, any cytotoxic (‘cyto’ means cell) agent can cause cancer and can be used to kill cancer cells (Figure 2). In fact, the first-ever chemotherapy was repurposed mustard gas, which was a cytotoxic chemical weapon used during World War I. Hence, modern chemotherapy is basically cytotoxic drugs with acceptable cancer specificity and benefit-harm ratio.
Figure 2. Various carcinogens (cancer-causing compounds), chemopreventive agents (drugs that prevent cancer development) and anti-cancer chemotherapeutic drugs (drugs that kill cancer cells) overlap as cytotoxic agents. Source: Blagosklonny (2005), Cell Death and Differentiation.
Unlike radiotherapy with site-dependent secondary cancer, chemotherapy-induced secondary cancer is mainly limited to leukaemia (blood cancer). Certain chemotherapeutic drugs can damage the DNA of blood-forming cells. Hence, the risk of secondary leukaemia is typically greater and develops faster after chemotherapy than radiotherapy, and the risk escalates further with increasing drug doses and treatment duration.
More recent evidence, however, indicates chemotherapy may also influence the risk of cancer other than leukaemia, especially in childhood cancer survivors. A large longitudinal study found that children treated with chemotherapy for sarcoma (connective tissue cancer) or leukaemia had a 5.3- and 4.1-fold greater risk of breast cancer in middle age. Other similar studies also noted increased risks of solid cancer, including breast cancer, among those who received chemotherapy during childhood. Experts speculate that children with more active cell division are more sensitive to DNA damage and the potential carcinogenic effects of chemotherapy.
Exacerbating the issue is combined chemotherapy and radiotherapy, i.e., radio-chemotherapy, which amplifies the risk of various secondary cancers by about 2-fold compared to one therapy modality alone. Additionally, while the risk of secondary cancer usually peaks at 5-9 years after chemotherapy, it can remain peaked for 25 years or more after radio-chemotherapy. However, despite the added toxicity from both radiotherapy and chemotherapy, radio-chemotherapy can be beneficial for certain types of metastatic cancer, particularly of the lungs, cervix, anal or bladder.
Preventive Strategies of Therapy-related Secondary Cancer
Although radiotherapy- and chemotherapy-induced secondary cancer are legitimate barriers to cancer care, it does not justify abandoning these potentially life-saving therapies. Rather, efforts should be made to develop strategies to minimise their adverse effects.
In this context, the role of complementary medicine becomes crucial. For example, the Pfeifer Protocol comprises powerful anti-cancer plant compounds, which also exhibit antioxidative and anti-inflammatory effects. Notably, some of these compounds can also alleviate the toxic effects of radiotherapy and chemotherapy, which may lower the risk of therapy-related secondary cancer.
Although the long-term effects of these plant compounds on the risk of secondary cancer have not been studied, their efficacy in mitigating the immediate toxicity of radiotherapy and chemotherapy is clearly evident. Minimising initial cell damage would also reduce the extent of DNA mutation and, consequently, the risk of secondary cancer. Therefore, these plant-based radioprotective and chemoprotective compounds offer a hopeful avenue for diminishing the long-term adverse effects of conventional cancer therapies.
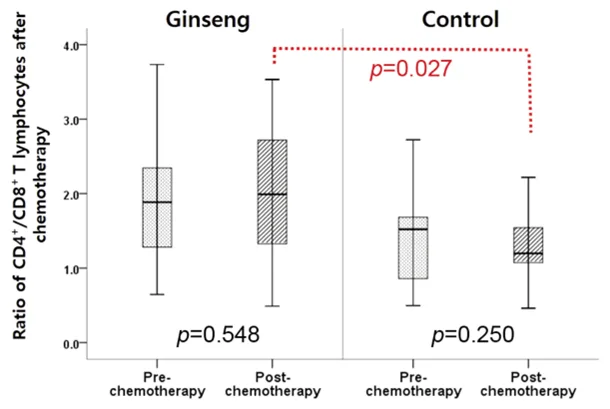
Figure 3. Cancer patients receiving ginseng had a significantly higher ratio of CD4+/CD8+ T cells (an indicator of improved immune function) after chemotherapy compared to the control group. Source: Kim et al. (2021), In Vivo.