An Introduction of Cancer Stem Cells (CSCs)
Why do normal cells transform into cancerous ones? The classic theory is that genetic mutations, particularly in genes that control cell growth and replication, drive cancer development. These mutations are often acquired throughout life due to eventual errors in DNA replication and exposure to carcinogens (e.g., smoking, viruses and radiation), but they can also be inherited in families with a history of cancer.
However, more recent research is recognising the role of cancer stem cells (CSCs), providing a more nuanced understanding of how cancers develop and persist. The existence of CSCs was first described in two landmark studies in 1994 and 1997 when Canadian scientists identified a small population of cells in leukaemia (blood cancer) that behaved differently from the bulk of the cancer. These cells were capable of self-renewal and differentiating into all the other cell types in the cancer, demonstrating properties similar to stem cells. While the origin of CSCs remains uncertain, scientists theorise that they may emerge from normal stem cells that acquire cancerous mutations or from cancer cells that gain mutations, endowing them with stem-like properties.
As studies expanded, CSCs have been identified in other cancer types, including those of the breast, colon, brain, skin and prostate, reinforcing the idea that cancer may stem from these CSCs. Remarkably, transplanting just a small amount of these CSCs (as few as 10 cells) was sufficient to trigger new leukaemia in mice. In contrast, conventional practice requires the transplant of a high number of cancer cells (thousands and even millions) to reproduce cancer in animal models.
Significance of CSCs in Cancer Care
The evolving understanding of CSCs is beginning to transform how we approach cancer treatment. One of the prevailing conundrums surrounding cancer is its unusually high rate of recurrence or relapse. Certain cancers, particularly brain and ovarian cancers, exhibit recurrence rates as high as 85-90% within a few years. Even cancers with lower recurrence rates still hover around 10-15%. These recurrence rates, however, have remained largely unaltered over the past decades despite the significant technological advancements in diagnosing and treating cancer – why?
A common explanation is that not all cancer cells are completely eradicated during treatment, leaving a small amount of residual cancer cells that may not be detectable. Another reason could be we have overlooked CSCs in conventional cancer therapy.
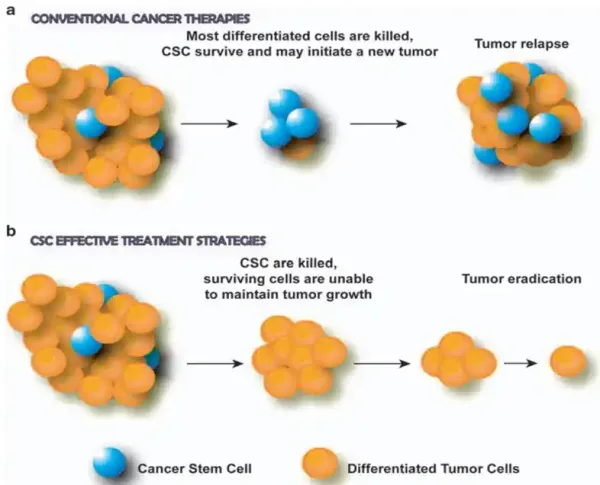
Due to their potent self-renewal and regenerative capabilities, CSCs are highly resistant to typical anti-cancer therapies. Their high survivability also enables CSCs to persist in distant organs and initiate new tumours, thus promoting cancer spread as well. Moreover, CSCs are highly adaptable; they can enter a dormant state to evade treatments that target rapidly dividing cells. In fact, the core principle of radiotherapy and chemotherapy is to destroy cancer cells that replicate more rapidly than normal cells. When such therapies are stopped, CSCs can re-enter the cell growth and replication cycle, driving cancer recurrence and spread (Figure 1A).
Figure 1. The role of cancer stem cells (CSCs) in tumour response to therapies. (a) Conventional cancer therapies kill differentiated (mature) cancer cells but spare CSCs, leading to tumour regrowth and recurrence. (b) CSC-targeting therapies destroy CSCs, preventing tumour recurrence and leading to tumour eradication due to the limited growth potential of remaining cells. Source: Eramo et al. (2010), Oncogene.
To make matters worse, recurrent cancers are usually more aggressive due to the CSCs retaining resistance to previously used chemotherapy or radiotherapy. The fear of cancer recurrence also poses substantial psychological distress for patients and their families. Additionally, patients experiencing cancer recurrence are more likely to face serious financial problems compared to those without recurrence, as they often require more expensive treatments. Therefore, many of the major challenges in cancer care – namely cancer recurrence and spread, therapy resistance and psychosocial distress – can be attributed to the notorious nature of CSCs.
As follows, targeting these pivotal cells could lead to more effective treatments that eradicate cancers at their root (Figure 1B). Emerging research has begun to stir in this direction, shedding light on several promising therapies, which we will explore in the next section.
Preliminary Therapeutics Targeting CSCs
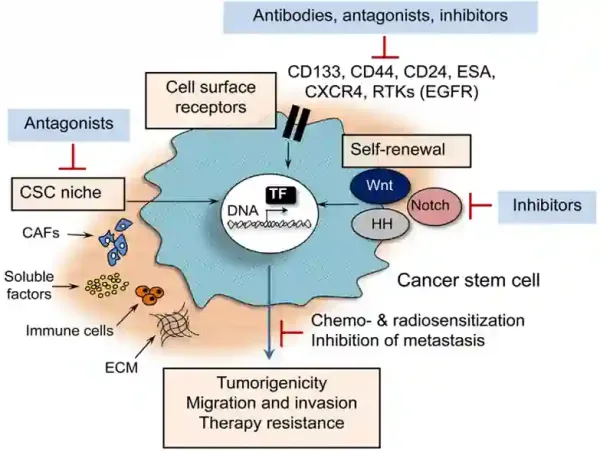
Current medical techniques targeting CSCs involve using antibodies designed to bind to molecules expressed on CSCs, such as CD44 and CD133, which are crucial for maintaining CSCs’ self-renewal properties. (CD stands for cluster of differentiation.) By binding to these molecules, antibodies can inhibit their activities or flag them for destruction by the immune system or cytotoxic agents. Other strategies involve targeting the CSC-associated signalling pathways (e.g., the Wnt, Notch and Hedgehog pathways) or cellular factors (e.g., growth factors, immune cells and fibroblasts) to disrupt processes essential for CSC maintenance and proliferation (Figure 2).
Some of these therapies are currently being investigated in ongoing clinical trials, primarily in phases I and II. However, none of these CSC-targeting therapies have been approved by the Food and Drug Administration (FDA) to date. While scientists are also exploring the repurposing of existing approved drugs to target CSCs, these efforts still require further validation in clinical trials. As a result, CSCs are unlikely to be targeted in conventional medicine as of now.
Figure 2. Current medical strategies that target cancer stem cells (CSCs). These include using (i) specific antibodies that bind to cell surface receptors of CSCs, such as CD133 and CD44; (ii) inhibitors of CSC signalling pathways, such as the Wnt, Hedgehog (HH) and Notch pathways; (iii) inhibitors of cellular factors in CSC niche, such as cancer-associated fibroblasts (CAFs), soluble factors (e.g., hormones and growth factors), immune cells and extracellular matrix (ECM). Source: Peitzsch et al. (2017), Seminars in Cancer Biology.
Fortunately, we are not entirely hopeless in fighting CSCs. Preliminary research has shown that certain plant extracts are capable of inhibiting the activities of CSCs in pre-clinical settings. For instance, a 2022 study from the U.K. screened over 10 compounds for their potential therapeutic activities against breast CSCs cultured in the laboratory. They found that two natural compounds (glucosamine and quercetin) and an FDA-approved anti-hypertension drug (carvedilol) managed to halt the self-replication of CSCs by disrupting their mitochondrial metabolism. In particular, quercetin, a flavonoid found in fruits and vegetables, is widely known for its potent antioxidant and anti-cancer activities.
Besides quercetin, other plant-derived compounds have also shown promise in inhibiting CSCs, as described in a 2020 review paper, titled “The ‘Big Five’ Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein.” Authored by scientists from Germany and America, this paper summarised the existing research on the effectiveness of these five plant compounds in targeting CSCs:
- Curcumin: Found in turmeric, curcumin has been found to suppress the self-renewal capabilities of CSCs of various cancers, including of the brain, colon, lung, breast and prostate, by interfering with the key signalling pathways of CSCs.
- Epigallocatechin-3-gallate (EGCG): Present in green tea, EGCG has been observed to sensitise prostate CSCs to apoptotic cell death by stopping their release of anti-apoptotic factors. Further studies reported that EGCG could eliminate CSCs and enhance the effectiveness of chemotherapy in mice with head and neck, colon and bone cancers.
- Sulforaphane: Similar to curcumin and EGCG, sulforaphane, abundant in cruciferous vegetables, has been found to inhibit CSCs by disrupting their signalling pathways and anti-apoptotic mechanisms in animal studies of breast, pancreatic and brain tumours.
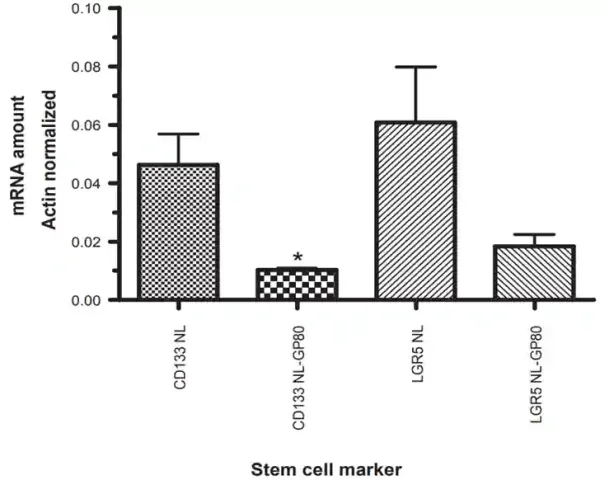
- Resveratrol: Derived from red grapes, resveratrol could differentiate brain and pancreatic CSCs into normal cancer cells, reversing their self-renewal properties and making them susceptible to chemotherapeutic drugs. Notably, a phase I clinical trial revealed that resveratrol suppressed the Wnt signalling pathway and decreased the viability of colon CSCs, as evident by the reduced expression of their surface markers (Figure 3).
- Genistein: Common in soy-derived foods, genistein is known to act on the hedgehog signalling pathway and CD44 receptor of CSCs to suppress tumour growth, a finding supported by animal studies of gastric, breast and prostate cancers.
“These five phytochemicals have recently gained enormous attention due to their ability to target CSCs by interfering with key signalling and survival pathways of CSCs, as clearly demonstrated in experimental studies and in xenograft mice with human cancer,” the review authors wrote. “Given their wide safety profile as naturally occurring and dietary phytochemicals with nearly no adverse effects, the five phytochemicals are well positioned as potent and specific agents for the elimination of CSCs in future clinical settings, alone, in combination with each other, and/or in combination with conventional cytotoxic drugs and novel cancer therapeutics.”
All these plant compounds, except sulforaphane, are already incorporated into the Pfeifer Protocol, a curated selection of plant compounds known for their diverse bioactivities, including anti-cancer. Our treatment protocols are tailored to individual patient needs, and recent research indicates that patients could also benefit from the CSC-targeting effects of these plant compounds. This approach aligns with our holistic treatment goals, which aim to complement the efficacy of conventional cancer therapies while helping to prevent cancer recurrence and spread.
Ultimately, as modern cancer care increasingly embraces CSC-targeting therapies, the inclusion of plant compounds in the Pfeifer Protocol that can inhibit CSCs adds a vital dimension to our treatment strategies in enhancing long-term patient care outcomes.
Figure 3. Expression of colonic stem cell markers CD133 and LGR5 before (NL) and after (GP80) treatment with resveratrol among participants with colon cancer in a phase I clinical trial. Results showed that resveratrol treatment reduced the expression of CD133 and LGR5 by nearly 3-fold. Source: Nguyen et al. (2009), Cancer Management and Research.