Introduction to Chemotoxicity and Chemobrain
While chemotherapy is undoubtedly a powerful weapon against cancer, it also has an unflattering reputation for causing various side effects – a double-edged sword, indeed. After all, the first-ever chemotherapy was a repurposed derivative of mustard gas, a toxic gas used during World War I. Thus, chemotherapy is fundamentally a cytotoxic (cyto- means cell) agent that kills rapidly dividing cells, such as cancer cells with uncontrolled growth rates. However, healthy cells also undergo division and replication, making them vulnerable to chemotherapy as well.
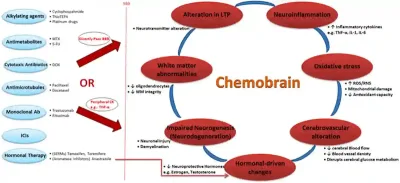
As a result, chemotherapy inadvertently harms healthy cells as collateral damage, which can affect multiple organ systems, such as the gastrointestinal, cardiovascular, renal (kidney), hepatic (liver), integumentary (skin, hair and nails), reproductive and nervous systems. It is not uncommon for cancer survivors to face long-term health effects (e.g., fatigue, nerve pain and cognitive problems) after chemotherapy. For instance, common chemotherapeutic drugs like docetaxel, 5-fluorouracil and methotrexate can cross the blood-brain barrier (BBB) or cause systemic inflammation that impair brain functions, resulting in long-term cognitive impairments known as the chemobrain (Figure 1). To make matters worse, such issues are often underdiagnosed and stigmatised as intellectual decline, laziness or part of the ageing process.
Although these potential toxicities may be necessary risks to treat cancer, it is crucial to dedicate more research to mitigating such chemotoxicity. To achieve this, we must better understand the underlying mechanisms of chemotoxicity. This article will explore the emerging discovery of how chemotherapy disrupts the gut microbiota and its subsequent effects on the brain via the microbiota-gut-brain axis, followed by promising mitigation strategies.
Figure 1. Mechanisms driving chemobrain or chemotherapy-induced cognitive impairments. Abbreviations/acronyms: 5-FU, 5-fluorouracil; BBB, blood-brain barrier; CK, cytokines; DOX, doxorubicin; ICIs, immune checkpoint inhibitors; LTP, long-term potentiation; MTX, methotrexate; ROS/RNS: reactive oxygen/nitrogen species; WM, White matter. Source: Mounier et al. (2020), Life Sciences.
How Chemotherapy Affects the Microbiota-Gut-Brain Axis
In pre-clinical settings, multiple animal studies have demonstrated that chemotherapy-induced inflammation is capable of disrupting both the gut microbiota makeup and cognitive functions. Experiments showed that transplanting the gut microbiota from chemotherapy-treated mice into healthy mice could reproduce a sustained pro-inflammatory state and behavioural signs of cognitive impairments, highlighting a cause-and-effect relationship between chemotherapy and disrupted microbiota-gut-brain axis. This axis, a two-way communication system between gut microbes and the brain, is widely implicated in health and diseases.
Although the effects of chemotherapy on the microbiota-gut-brain axis have been historically understudied in clinical settings, recent research is starting to bring this critical issue to light. In a newly published study, scientists at the U.S. Ohio State University found that chemotherapy survivors showed signs of gut microbiota disruptions that correlated with cognitive decline. Specifically, they collected stool samples, blood and cognitive test results from 77 breast cancer patients before, during and after chemotherapy. Results revealed that the patients’ gut microbiota began to lose microbial diversity during chemotherapy treatment, which did not return to the pre-chemotherapy state even months after the treatment ended. This loss in gut microbiota diversity was also correlated with increased levels of pro-inflammatory cytokines in the blood and reduced cognitive functions, corroborating the inflammatory effects of chemotherapy on the microbiota-gut-brain axis. A similar loss in gut microbiota diversity was also previously reported in patients with lymphatic, colorectal or ovarian cancer who underwent chemotherapy.
“We found that patients treated with chemotherapy who showed decreases in cognitive performance also had reductions in the diversity of their gut microbiome,” said Dr Leah Pyter, PhD, an associate professor of psychiatry and neuroscience at Ohio State University, who directed the new study. However, such side effects are frequently dismissed as “part of chemotherapy,” Dr Pyter lamented, resulting in the lack or absence of treatment efforts.
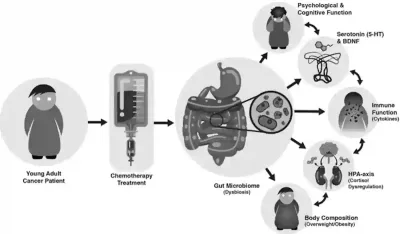
These recent findings also align with prior clinical observations. Gastrointestinal side effects, such as diarrhoea and vomiting, affect up to 80% of patients undergoing chemotherapy and are often accompanied by unfavourable shifts in gut microbial populations. Additionally, chemotherapy-induced cognitive impairment (i.e., chemobrain) is a pervasive yet under-recognised problem in cancer care, affecting as many as 75% of patients. For example, a longitudinal study found that women who received chemotherapy for early-stage breast cancer continued to exhibit signs of cognitive impairments more than 20 years later, compared to healthy women of similar age. Combining these observations, it is obvious that chemotherapy disturbs the microbiota-gut-brain axis, which scientists warn could lead to further detrimental effects (Figure 2).
The microbiota-gut-brain axis influences the immune and hormonal systems. Certain species of gut bacteria produce anti-inflammatory metabolites, such as butyrate and propionate, from the digestion of fibres. However, the cytotoxic effects of chemotherapy can kill these beneficial gut bacteria. This situation creates an opportunity for pathogenic microbes to overgrow in the gut, causing damage to the intestinal barrier and leakage of harmful substances into the bloodstream (i.e., leaky gut), further triggering a systemic pro-inflammatory immune response.
Moreover, gut microbes release neurotransmitters like serotonin and glutamate, which act on the vagus nerve to regulate neurological functions and the hypothalamic-pituitary-adrenal (HPA) axis in the brain. The HPA axis, a stress response system, produces cortisol and other stress-related hormones to prepare the body for a fight-or-flight state. Aggravating the situation, inflammatory signals can trigger the HPA axis, which then feeds back into the immune system to exacerbate inflammation levels, creating a vicious cycle. Therefore, disruption of the microbiota-gut-brain axis, whether due to chemotherapy or other factors, is closely tied to chronic inflammation and overactive stress response, as well as various physical and mental ailments (Figure 2).
Figure 2. Chemotherapy-induced disruption of the microbiota-gut-brain axis. This model suggests that chemotherapy induces long-term gut dysbiosis, increasing intestinal permeability and allowing bacterial toxins to escape into the bloodstream. This triggers a feedback loop of increased systemic inflammation and dysregulated HPA axis. These conditions also reduce serotonin (5-HT) and brain-derived neurotrophic factor (BDNF) levels, leading to psychiatric and cognitive issues. Additionally, these disruptions may promote adipose tissue accumulation, contributing to obesity and other metabolic diseases. Source: Deleemans et al. (2023), BMC Cancer.
Strategies to Mitigate Chemotoxicity
Based on the above, research into finding ways to reduce the toxic effects of chemotherapy is paramount to preventing long-term health issues and poor quality of life. Current medical practices tend to overemphasise life expectancy over life quality. As follows, standard medical guidelines have not established protocols to mitigate the chemotoxicity effects on the microbiota-gut-brain axis. However, preliminary research efforts have explored a few promising alternatives: probiotics, faecal microbiota transplantation (FMT) and plant compounds.
First, probiotics are live microbes that confer health benefits when consumed in adequate amounts. Randomised clinical trials have reported that probiotics could prevent chemotherapy-induced inflammation and diarrhoea in patients with lung, blood or colorectal cancer, although the precise effects on the microbiota-gut-brain axis were not investigated. However, pre-clinical experiments with animals have shown that early probiotic treatment can prevent gut microbiota disruptions, intestinal barrier damage and cognitive deterioration following chemotherapy, highlighting the protective effects of probiotics on the microbiota-gut-brain axis.
Second, FMT refers to a procedure that transfers stool from a healthy donor into the gastrointestinal tract of a recipient to restore a balanced gut microbiota. FMT is typically performed via non-surgical means, such as a colonoscopy inserted through the rectum. Similar to probiotics, pre-clinical studies have demonstrated that FMT can attenuate gut microbiota disruptions and intestinal inflammation caused by chemotherapy. One clinical trial reported that FMT restored the gut microbial diversity after chemotherapy-induced disruptions among patients with blood cancer; FMT also reduced systemic levels of oxidative stress and inflammation in this study.
Third, plant compounds with bioactive properties are used in the field of phytotherapy to enhance overall health, prevent diseases and complement the effectiveness of standard medical therapies. Our Pfeifer Protocol capitalises on phytotherapy to provide personalised treatment tailored to each patient’s needs, with the primary goal of improving the quality of life for cancer patients, including those suffering from therapy-related side effects. Certain compounds in the Pfeifer Procol have been shown to mitigate chemotoxicity, including its detrimental effects on the gut microbiota and cognitive functions:
- Curcumin (from turmeric): Two separate studies from China and Brazil in 2020 and 2021 reported that curcumin restored the cognitive functions of chemotherapy-treated rodents by stimulating the cell’s autophagy (self-cleaning) system to remove inflammatory cell components, thereby reducing inflammation in the brain (Figure 3). Another study from the U.S. showed that curcumin could support the growth of beneficial bacteria in the gut of mice with cancer, which helped slow tumour progression and improve survival.
- Resveratrol (from grapes and berries): The Brazillian study also examined resveratrol, demonstrating its ability to prevent memory deterioration in chemotherapy-treated mice by reducing neuroinflammation (Figure 3). Similar findings were previously replicated in a 2018 study from Hong Kong. Other research also supports the ability of resveratrol to enhance gut microbiota diversity and maintain intestinal barrier integrity.
- Ginsenosides (from ginseng): According to research from China and Egypt, ginsenosides are highly effective in reversing the harmful effects of chemotherapy on the gut microbiota, intestinal barrier and brain functions of rodents, owing to its potent antioxidative and anti-inflammatory effects.
- Others: Arabinoxylan from rice brain, indole-3-carbinol from cruciferous vegetables, quercetin from onions and fruits, and citrus pectin from fruit peel are also beneficial plant compounds for the gut microbiota, although their therapeutic effects against chemobrain requires further research.
Overall, these natural plant compounds, alongside probiotics and FMT, have shown promising potential in regulating the microbiota-gut-brain axis to prevent or alleviate symptoms of chemobrain. Notwithstanding the importance of chemotherapy as an anti-cancer treatment, its adverse impacts on the gut microbiota and brain health underscore the need for effective mitigation strategies. Ensuring cancer survivors can lead healthier, more fulfilling lives post-treatment is essential. At Integrative Cancer Care, we advocate for a medical approach that prioritises not only extending life expectancy but also enhancing the quality of life through holistic care.
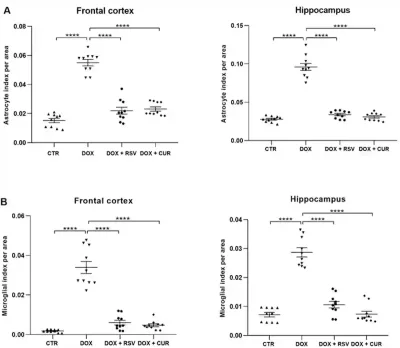
Figure 3. Effects of resveratrol (RSV) and curcumin (CUR) in alleviating brain inflammation induced by the chemotherapeutic agent, doxorubicin (DOX). Brain inflammation was measured by microglial activities in the frontal cortex (A) and hippocampus (B). The frontal cortex is crucial for executive processing (e.g., planning and problem-solving), while the hippocampus stores memories. Source: Moretti et al. (2021), Research in Veterinary Science.