Peto’s Paradox
Despite not holding a doctorate, Sir Richard Peto, now 81 years old, is a highly distinguished scientist. His scientific contributions have earned him numerous accolades, including a knighthood from Queen Elizabeth II, Fellowship of the Royal Society (FRS), honorary PhD from Yale University, and professorship at the University of Oxford. Specifically, Sir Peto is renowned for pioneering work in developing meta-analyses, establishing the causal link between smoking and lung cancer, and inspiring the concept of Peto’s Paradox.
It all began in a 1977 paper in which Sir Peto highlighted a perplexing observation: given that cancer is primarily driven by mutations in our cells, one would expect that large, long-lived humans would have a significantly higher risk of developing cancer compared to small, short-lived animals like mice. In reality, however, the cancer rates between humans and mice are not drastically different. Sir Peto questioned how human cells are not significantly more prone to cancer than mouse cells, despite having a much greater potential for mutations. He proposed there must be an evolutionary factor that enables humans to grow larger and live longer without being disproportionately affected by cancer (Figure 1).
Figure 1. A snippet of the seminal 1977 paper by Richard Peto, whose ingenious writings and observations highlight the paradox between cancer rate and body size across species. Source: Peto (1977), Cold Spring Harbor Laboratory Library.
Subsequent research has reinforced Sir Peto’s observation that cancer rates do not correlate with body size across species, giving rise to Peto’s Paradox. For instance, elephants and whales exhibit surprisingly low cancer rates despite their immense size. Unravelling the mechanisms behind their cancer resistance could provide valuable insights into cancer suppression or prevention. In this newsletter, we will delve into Peto’s Paradox and what it reveals about cancer biology.
Why it is a Paradox
In multicellular organisms, cells need to grow and divide regularly. Each time a cell divides, it must copy its DNA, but this process is imperfect and can create mutations. Some of these mutations can affect important genetic pathways that control cell cycle and DNA repair, which can lead to uncontrolled cell growth, i.e., cancer. Since every cell division carries a chance of creating a cancerous mutation, you would expect that cancer risk increases with the number of cell divisions an organism undergoes in its lifetime. This means that larger and longer-lived animals should theoretically have a higher risk of cancer.
This concept holds true within a species, where cancer rate is generally proportional to the number of cells. For example, a 1998 study tracked 17,738 British civil servants over 25 years and found a strong positive correlation between height and cancer incidence, even after adjusting for other risk factors like smoking and age. More recently, a meta-analysis calculated that each 10 cm increase in height was associated with a 10-14% increase in cancer incidence in humans. This meta-analysis synthesised data from 11 studies across continents, further solidifying height as a universal cancer risk factor. Similar trends are observed in dogs, where larger breeds are more prone to cancer than smaller breeds. These findings suggest that height or body size – both indicators of cell number – does indeed correlate with cancer risk within a single species.
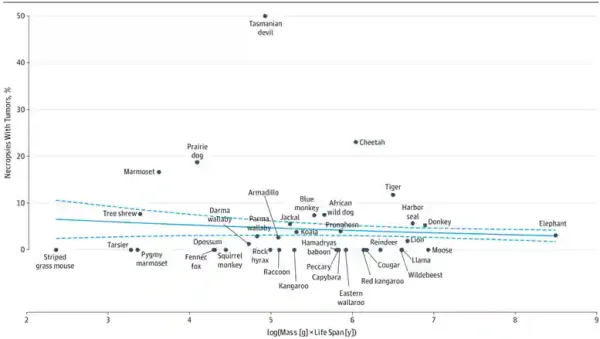
Between species, however, the relationship becomes murky. In a landmark 2015 study, Abegglen et al. from the Huntsman Cancer Institute, US, analysed the presence of cancer in autopsy tissues across 36 mammalian species, ranging from 51-gram striped grass mice to the massive 4,800-kilogram elephant. They found no link between an animal’s body size and its likelihood of getting cancer, supporting Sir Peto’s observation that bigger animals are not necessarily more susceptible to cancer (Figure 2). In fact, the evidence suggests that larger, long-lived mammals may actually develop cancer less frequently. Such findings bear crucial implications for our understanding of how nature has evolved solutions to overcome cancer.
Figure 2. Cancer incidence across species by body size and life span. Larger and longer-lived species are not any more susceptible to cancer than smaller, shorter-lived species. In fact, there may even be a trend towards lower cancer rates in larger, longer-lived species. Source: Abegglen et al. (2015), Journal of American Medical Association (JAMA).
What Resolves the Paradox
Abegglen et al. were then fascinated by the remarkable cancer resistance of elephants, whose cancer rate is only 4-5% compared to that of humans at 10-25%. When they analysed the genome of elephants, Abegglen et al. found at least 20 copies of the TP53 gene compared to only one copy in humans. TP53 is a well-known tumour suppressor gene that induces apoptotic cell death of mutated cells when DNA repair mechanisms fail. Further experiments showed that the TP53 gene activities in elephant cells are more active and effective in preventing cancer development compared to human cells when exposed to ionising radiation.
A more recent study from the University of Chicago, U.S., also found 20 active copies of the TP53 gene in elephant’s genome. The increase in TP53 copies also coincided with the evolution of large body sizes and enhanced resistance to DNA-damaging stressors. The importance of this gene is further exemplified in genetic disorders, such as Li-Fraumeni syndrome. Individuals born with this condition have one faulty TP53 gene and face over 70% (for men) and 90% (for women) lifetime risk of developing multiple cancers. Imagine elephants with 20 copies of those genes!
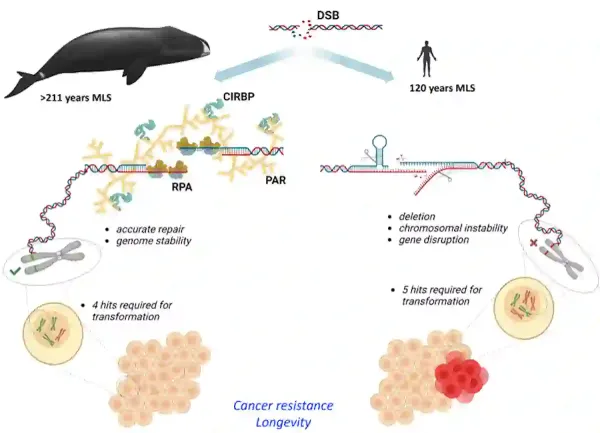
A 2023 study also builds upon Peto’s Paradox by investigating the bowhead whale, the longest-living and highly cancer-resistant mammal, which can weigh over 80,000 kg and live for more than 200 years. Conducted by Firsanov et al. at the University of Rochester, U.S., the research analysed gene expression and cell behaviour in these whales. Results revealed that whale cells have a highly efficient mechanism for repairing DNA damage compared to other mammals. This superior DNA repair capability is linked to the elevated expression of certain proteins, notably CIRBP (cold-inducible RNA-binding protein) and RPA2 (replication protein A2) (Figure 3).
Additionally, Firsanov et al. discovered that the TP53 gene activities in whale cells are even lower than in other mammals, as well as that whale cells require fewer mutations to turn cancerous. This indicates that the whale’s cancer resistance mainly arises from its exceptional DNA repair capacity. In this sense, whales may have a more effective cancer-resistant strategy compared to elephants. Elephants rely on eliminating mutated cells, which could deplete stem cells and facilitate ageing-related degeneration. In contrast, whales focus on repairing mutations, thereby preserving DNA integrity and contributing to disease-free longevity (Figure 3).
“By studying a mammal capable of maintaining its health and avoiding death from cancer for over two centuries,” Firsanov et al. wrote in their 2023 paper, “we are offered a unique glimpse behind the curtain of a global evolutionary experiment that tested more mechanisms affecting cancer and ageing than humans could ever hope to approach.”
Figure 3. A graphical summary of the cancer resistance and longevity of bowhead whales. Bowhead whales have an exceptionally efficient mechanism to repair DNA damage, thanks to high levels of certain proteins like CIRBP and RPA2. This advanced DNA repair system likely helps them resist cancer, even though it takes fewer genetic changes to make whale cells turn cancerous compared to human cells (i.e., 4 vs. 5 hits). Source: Firsanov et al. (2023), bioRxiv.
What Can We Learn From Solving the Paradox
As we discussed, elephants demonstrate remarkable cancer resistance due to having multiple copies of the TP53 gene. What happens if we increase the expression of TP53 artificially? This was attempted with laboratory mice, but initial efforts were discouraging: while the mice did become more resistant to cancer, they aged more rapidly, and their organs degenerated faster. Scientists then refined their approach, carefully controlling TP53 activities to prevent overexpression to strike a balance between cancer suppression and maintaining longevity. Efforts are also underway to target TP53 in humans, with the goal of achieving similar benefits.
Remember CIRBP and RPA2? These cancer-resistant proteins are not unique to whales, although they are normally expressed at low levels in other mammalian species. As follows, Firsanov et al. postulate that targeting CIRBP and RPA2 could help enhance DNA repair activities in humans. In particular, CIRBP is a cold-inducible protein, which explains its exceptionally high expression in whales that inhabit freezing waters. This aligns with the known health benefits of cold exposure, such as cold-water immersion, which is commonly used in sports medicine to reduce inflammation and speed up recovery after injuries or intense training. However, evidence remains lacking on whether regular cold exposure could prevent cancer or extend longevity. Even so, the discomfort of cold exposure may deter individuals from incorporating it into their routine.
Alternatively, efforts could be directed toward designing new drugs or exploring natural compounds that augment the expression of CIRBP and RPA2. For example, a 2017 study from Johannes Gutenberg University in Germany found that co-administering vitamin C and curcumin can enhance DNA repair activities, including RPA2, as part of their anti-cancer effects against laboratory-cultured cancer cells. As Firsanov et al. concluded, “Therapeutics based on increasing the activity or abundance of proteins like CIRBP or RPA2 could one day enable the treatment of genome instability as a modifiable disease risk factor.”
By drawing insights from nature’s evolutionary solutions in long-lived mammals, we may one day unlock new methods to extend human longevity and combat cancer more effectively. The journey to understand and apply these lessons is just beginning, offering hope for breakthroughs that could transform how we approach ageing and disease.