Generations of scientists have devoted their careers to decoding the complex interactions between the immune system and cancer. Early pivotal moments include the first observation of cancer disappearance after an infection in 1868 and the first immunotherapy attempt at using bacterial toxins to stimulate the immune system to kill cancer in 1891. Subsequent efforts gradually paved the way for the immunotherapy methods we see today, such as immune checkpoint inhibitors, engineered immune cells, cancer vaccines, cytokine therapy and monoclonal antibodies. These methods fundamentally rely on boosting the immune system’s abilities to target and destroy cancer cells.
Immunotherapy is now recognised as the ‘fourth pillar’ of standard cancer treatment, alongside surgery, radiation and chemotherapy. The advent of immunotherapy has greatly benefited patients by providing an alternative treatment option for difficult-to-treat cancers. These include recurrent, metastasised (already spread) or treatment-resistant cancers, where more traditional approaches of surgery, chemotherapy and radiotherapy proved to be less or not effective.
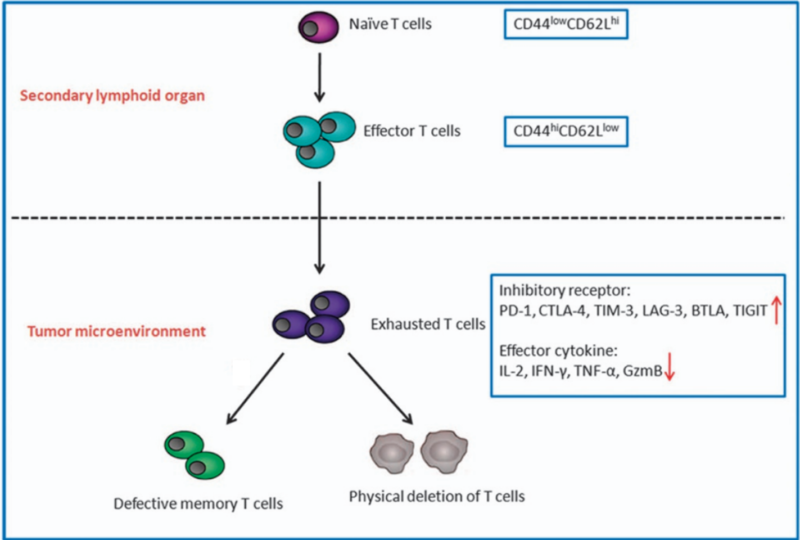
Despite that, immunotherapy still faces certain challenges. Most forms of immunotherapy rely on boosting the anti-cancer capacity of cytotoxic T-cells, a type of immune cell that kills rogue cells, such as virus-infected or cancerous cells. However, in the context of persistent infection or cancer, these T cells are constantly activated and eventually lose their potency, entering a state of functional exhaustion. In this exhausted state, the T cells start showing high levels of inhibitory signals, most notably programmed cell death protein 1 (PD-1), which act as ‘brakes’ on their activity. Their production of anti-cancer cytokines like granzyme B and interferon-gamma also decreases, leading to an overall reduction in anti-cancer immune response (Figure 1).
T-cell exhaustion is a major factor limiting the effectiveness of current immunotherapy. Even treatments designed to block these inhibitory signals (e.g., PD-1 checkpoint inhibitors) often cannot fully restore T-cells to their fresh state. However, the relatively recent discovery of a subset of T-cells, called progenitor exhausted T (Tpex) cells, may soon help scientists overcome the hurdles of T-cell exhaustion and design more effective immunotherapy strategies.
Figure 1. T-cell exhaustion in the tumour microenvironment. Naïve T-cells first differentiate into effector T-cells in the thymus, a secondary lymphoid organ. In the tumour, T-cells may become exhausted if repeated efforts to eradicate the tumour are unsuccessful. Exhausted T-cells exhibit increased inhibitory receptors and decreased production of anti-cancer cytokines, eventually leading to defective and depleted T-cells. Source: Jiang et al. (2015), Cell Death and Disease.
Over the past decade, studies involving animal models or human cases of persistent infection and cancer have found that the immune system has a reserve pool of T-cells. However, this reserve is finite and only becomes actively engaged when T-cells have become exhausted and depleted. By the time this reserve is tapped into, the disease often has advanced to a stage where the immune system can only contain, not eradicate, the disease. Imagine if immunotherapy strategies could access this reserve pool faster to eliminate cancer before it progresses. To achieve this, we need to deepen our understanding of how these reserve T-cells function and the mechanisms governing their activation.
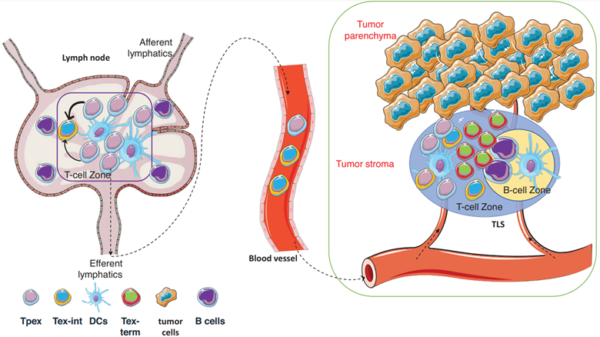
To this end, scientists have focused on Tpex cells, characterised by a high expression of a transcription factor called T-cell factor 1 (TCF-1). Located in the lymph nodes, Tpex cells possess stem-like qualities that can self-renew and differentiate into functional T-cells (Figure 2). While these regenerated T-cells remain in an exhausted state (i.e., intermediate exhausted T-cells), they are less defective than the existing pool of exhausted T-cells (i.e., terminally exhausted T-cells). The self-renewal capacity of Tpex cells is precious because it provides a continuous supply of T-cells to sustain long-term immune responses, especially against persistent threats like cancer.
Research has found that dysfunctional T-cell response in human cancer is not due to the build-up of exhausted T-cells but the inadequate activation of Tpex cells. Indeed, a higher proportion of Tpex cells has been correlated with better clinical outcomes in cancer patients, even in advanced stages of the disease. Interestingly, the success of PD-1 inhibitors (i.e., a widely used type of immune checkpoint inhibitor in immunotherapy) is now primarily attributed to their ability to activate Tpex cells rather than merely preventing T-cell exhaustion.
Figure 2. Activation of Tpex cells in cancer. In the tumour-draining lymph nodes, the resident Tpex cells can migrate to the tumour and differentiate into new T-cells that are less exhausted. Abbreviations: Tpex, progenitor exhausted T-cells; Tex-int, intermediate exhausted T-cells; DCs, dendritic cells; and Tex-term, terminally exhausted T-cells. Source: Ni (2024), Clinical and Translational Medicine.
Tpex cells are also integral to establishing long-term immune memory, the immune system’s ability to ‘remember’ previous encounters with pathogens or tumours and respond more effectively upon re-exposure. With their stem-like properties, Tpex cells can continuously regenerate memory T-cells that remain vigilant of any cancer recurrence. After initial cancer therapies, a small number of residual tumour cells often persist, which may proliferate and cause a relapse years later. Tpex cells, through their continuous supply of functional T-cells, help eliminate these residual tumour cells that exhausted T-cells may overlook. As a result, Tpex cells are not only vital in preventing T-cell exhaustion but also in preventing cancer recurrence and achieving long-term remission.
“Tpex cells significantly shape the response to immunotherapy, thereby emphasising the crucial role of augmenting Tpex cell proportions within the [tumour microenvironment] to increase therapeutic efficacy,” stated a 2024 review paper in Cancer Biology and Medicine. “Consequently, future endeavours in drug development and delivery strategies targeting Tpex cells will be crucial avenues for augmenting the efficacy of immunotherapy.”
Since Tpex cells are distinguished by their high expression of TCF-1, scientists have experimented with artificially increasing TCF-1 levels in exhausted T-cells. Early findings in mice suggest that this approach can restore these T-cells to a more stem-like state, thereby improving their ability to control tumour growth. As current immunotherapy already involves genetically engineering T-cells to express tumour-specific receptors, such as in chimeric antigen receptor (CAR) T-cell therapy, the same technology may be applied to enhance TCF-1 expression—a promising strategy that warrants further clinical research.
Emerging research suggests that Tpex cells are regulated by other cellular factors. For instance, a 2023 study from the University of Basel, Switzerland, found that interleukin-33 (IL-33) could enhance the expansion and self-renewing activities of Tpex cells by preventing the loss of TCF-1 expression in animal models. IL-33 is an anti-inflammatory cytokine with protective roles against diseases. Interestingly, certain natural plant compounds, namely curcumin and ginseng, have been shown to enhance IL-33 expression in preclinical studies, suggesting that these compounds could also strengthen Tpex cell function and overall immune responses.
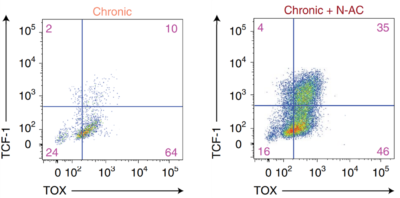
In addition, oxidative stress is known to drive T-cells toward terminal exhaustion. Studies have demonstrated that antioxidant intervention with N-acetylcysteine (NAC) supplementation can improve mitochondrial health, cytokine production and self-renewal properties of T-cells. Specifically, NAC treatment managed to recover TCF-1 expression, essentially reinvigorating their Tpex state, in up to half of the exhausted T-cells (Figure 3). Such benefits were also replicated with another antioxidant agent, a synthetic vitamin E called Trolox. These findings highlight the importance of managing mitochondrial oxidative stress to support Tpex cell function.
Figure 3. Flow cytometry showing the expression of TCF-1 and TOX in chronically stimulated T-cells (exhausted) with and without NAC treatment. A higher proportion of cells began to express TCF-1 and TOX following NAC treatment. While TCF-1 is a Tpex cell marker, TOX also plays an important role in preserving the stem-like qualities of Tpex cells. Abbreviations: TCF-1, T-cell factor 1; TOX, thymocyte selection-associated high mobility group box protein. Source: Vardhana et al. (2022), Nature Immunology.
Antioxidants are widely revered for their numerous health properties, including recent findings that show their role in supporting Tpex cells. A vast resource of antioxidants can be found in the plant kingdom, as plants have evolved potent antioxidant capacity to counteract environmental stressors, such as sunlight and drought. This underscores the value of adopting a plant-based diet and even incorporating plant-derived compounds into our lifestyle. For this purpose, the Pfeifer Protocol applies therapeutic plant compounds to provide personalised, complementary treatments that enhance the quality of life of cancer patients. By combining modern immunotherapy with plant-derived antioxidants, this integrative approach paves the way for more effective cancer care strategies that improve patient outcomes and support long-term immune resilience.
Although no therapies currently target Tpex cells directly, ongoing research into the mechanisms governing their regulation is a source of inspiration for developing more effective treatments in the future. The discovery that mitigating oxidative stress preserves Tpex cells further showcases the profound connection between lifestyle choices and immune health. It is compelling that even advanced immunological findings are still connected to basic health principles, emphasising the impact of holistic well-being on disease prevention and treatment.