Chimeric Antigen Receptor (CAR) T-cell Therapy: A Game-Changer in Cancer Care
Introduction: A Brief History
The discovery of immunotherapy was so meaningful that it was named the “Breakthrough of the Year” in 2013 by Science, the leading scientific journal in the U.S. Immunotherapy is a unique form of cancer therapy, which capitalises on the body’s immune system to attack the tumour or cancer cells. This is in contrast with other existing cancer therapies, which tend to overly rely on external agents, such as chemotherapeutic drugs and radiation.
Immunotherapy originated in the 1860s, when German physicians noticed that some cancer patients with skin infections experienced tumour regression. They cleverly deduced that immune responses directed against the infection might have cross-reacted with the cancer. Subsequent efforts witnessed the deliberate infection of Streptococcus pyogenes and Serratia marcescens (bacteria that causes skin infection) into cancer patients, albeit with mixed results. However, setbacks are valuable feedback that fuels further innovation and progress.
Thankfully, present-day immunotherapy does not entail deliberately inoculating patients with potentially dangerous infectious agents. Modern technology has successfully imitated such anti-cancer immune responses with medications.
Principles of Current Immunotherapy
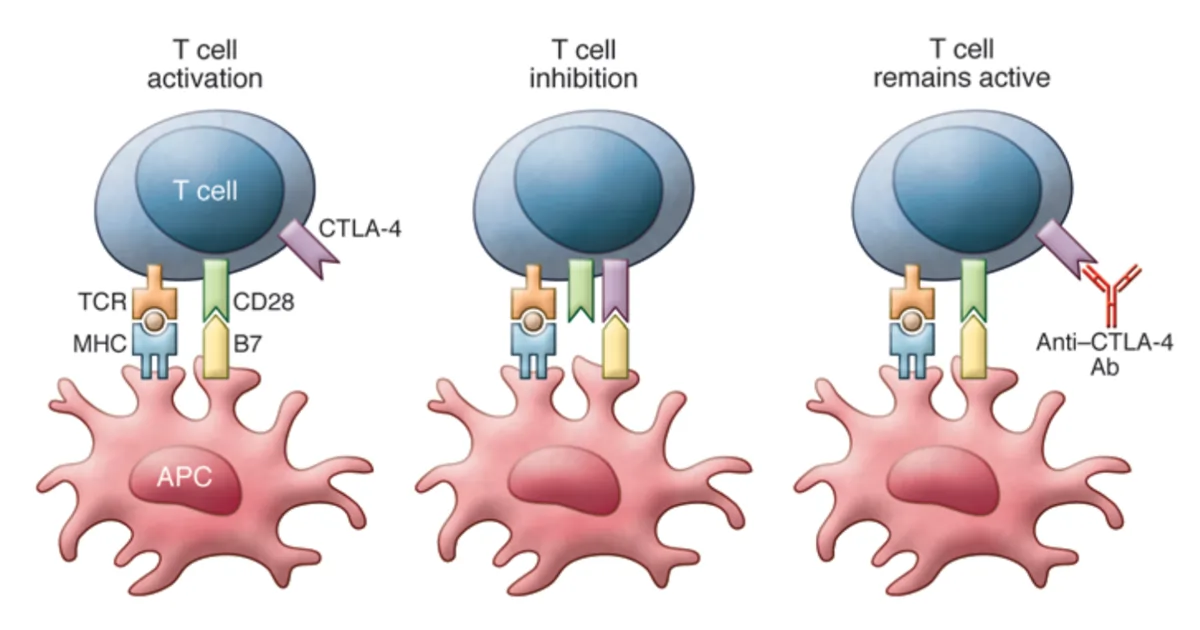
Modern immunotherapy has become a reality owing to the breakthrough discovery of immune checkpoint inhibitors. T-cells of the adaptive immune system express checkpoint proteins, such as cytotoxic T lymphocyte antigen 4 (CTLA-4), which suppress their activities when necessary (Figure 1). This regulatory mechanism is like an off-signal, preventing T-cells from going hyperactive, as seen in certain autoimmune diseases.
A major class of T-cells are cytotoxic T-cells, which destroy abnormal cells, such as virus-infected or cancerous cells. As follows, scientists found that using specific antibodies to block CTLA-4 switches off the off-signal of T-cells, thereby allowing their cytotoxic activities to continue attacking the tumour (Figure 1). In 2010, ipilimumab was the first CTLA-4-blocking antibody approved by the Food and Drug Administration (FDA) to treat melanoma (skin cancer).
Afterwards, more immune checkpoint inhibitors were successfully developed and approved for cancer therapy. These include inhibitors of programmed death-1 (PD-1) (e.g., nivolumab and pembrolizumab) and PD-1 ligand 1 (PD-L1) (e.g., avelumab and durvalumab), approved for treating skin-, liver-, lung- and other cancers.
Figure 1. Mechanism of T-cell activation and inhibition with antigen-presenting cells (APC). Left: T-cell activation requires co-stimulation through T-cell receptor (TCR, brown) and cluster of differentiation 28 (CD28, green). Middle: T-cell inhibition requires the activation of cytotoxic T lymphocyte antigen 4 (CTLA-4, purple). Right: T-cell remains active if CTLA-4 is blocked with anti-CTLA-4 antibodies (Ab). Source: Buchbinder and Hodi (2015), Journal of Clinical Investigation.
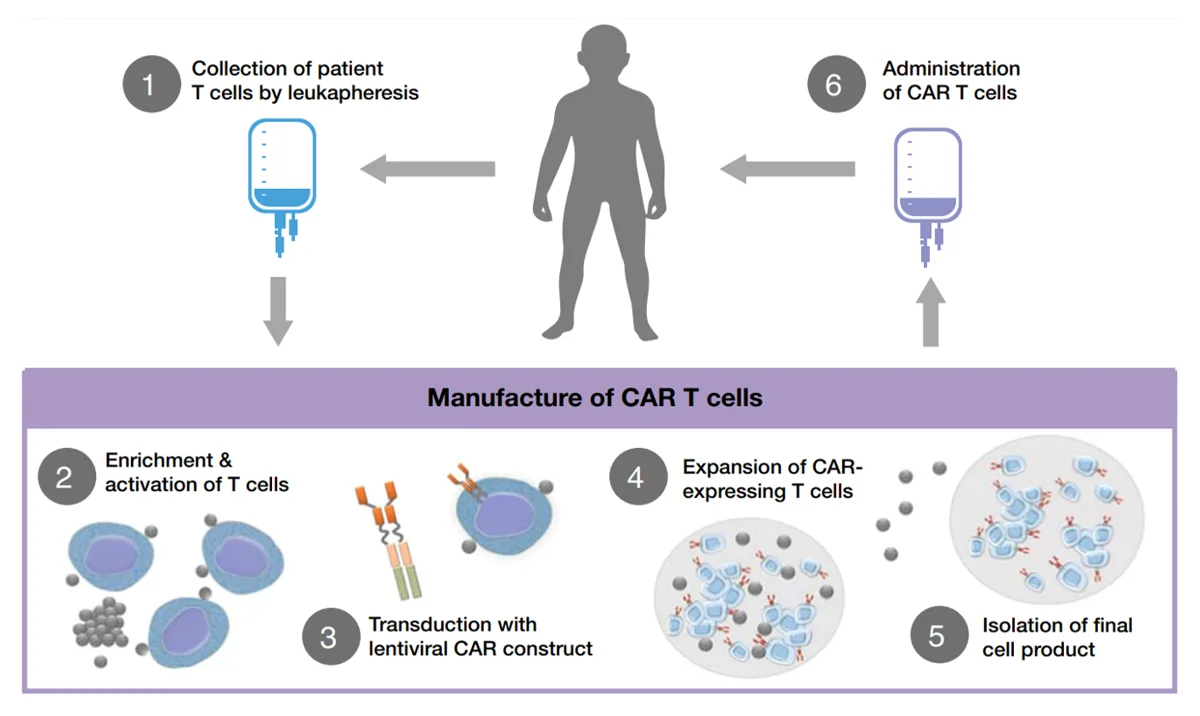
More recently, an innovative form of immunotherapy, i.e., chimeric antigen receptor (CAR) T-cell therapy, has emerged. Instead of targeting existing checkpoint molecules, CAR T-cell therapy engineers a patient’s T-cells to express CARs. This process begins with isolating the patient’s T-cells, which are then genetically modified to express CARs and subsequently re-infused into the patient (Figure 2). Such CARs can be designed to recognise and bind to specific proteins on cancer cells. Upon binding, CAR-expressing T-cells began to multiply and release cytokines to kill and flag the cancer cells for destruction. Alternatively, CAR T-cells can also release toxic granules that induce apoptotic cell death in the targeted cancer cells.
In 2017, the FDA approved tisagenlecleucel, the first CAR T-cell therapy for the treatment of leukaemia (blood cancer). A single infusion of tisagenlecleucel achieved a remarkable 81% remission rate within three months in a landmark clinical trial involving children and young adults with leukaemia.
Later, more forms of CAR T-cell therapy also gained FDA approval for various types of leukaemia and melanoma after showing decent successes in clinical trials.
Figure 2. The general process of chimeric antigen receptor (CAR) T-cell therapy. Source: Hucks and Rheingold (2019), Blood Cancer Journal.
Challenges and Potential of CAR T-cell therapy
No therapy is perfect, however. One caveat is that CAR T-cells may release excessive pro-inflammatory cytokines, triggering cytokine release syndrome (CRS) or, worse, immune cell-associated neurotoxicity syndrome (ICANS) in some patients (Figure 3). In CRS, high levels of pro-inflammatory cytokines can cause fever, fatigue, pain and other symptoms. If such cytokines further damage the blood-brain barrier and brain, it can cause ICANS with symptoms of severe cognitive impairments, delirium and seizure.
However, both CRS and ICANS are manageable with timely administration of standard anti-inflammatory therapies, such as tocilizumab or corticosteroids. In clinical trials investigating CAR T-cell therapy, CRS and ICANS only lasted a few days with near-zero fatalities. Of the numerous clinical trials, only one of them documented a single death (out of 17 patients with melanoma) due to CRS associated with CAR T-cell therapy.
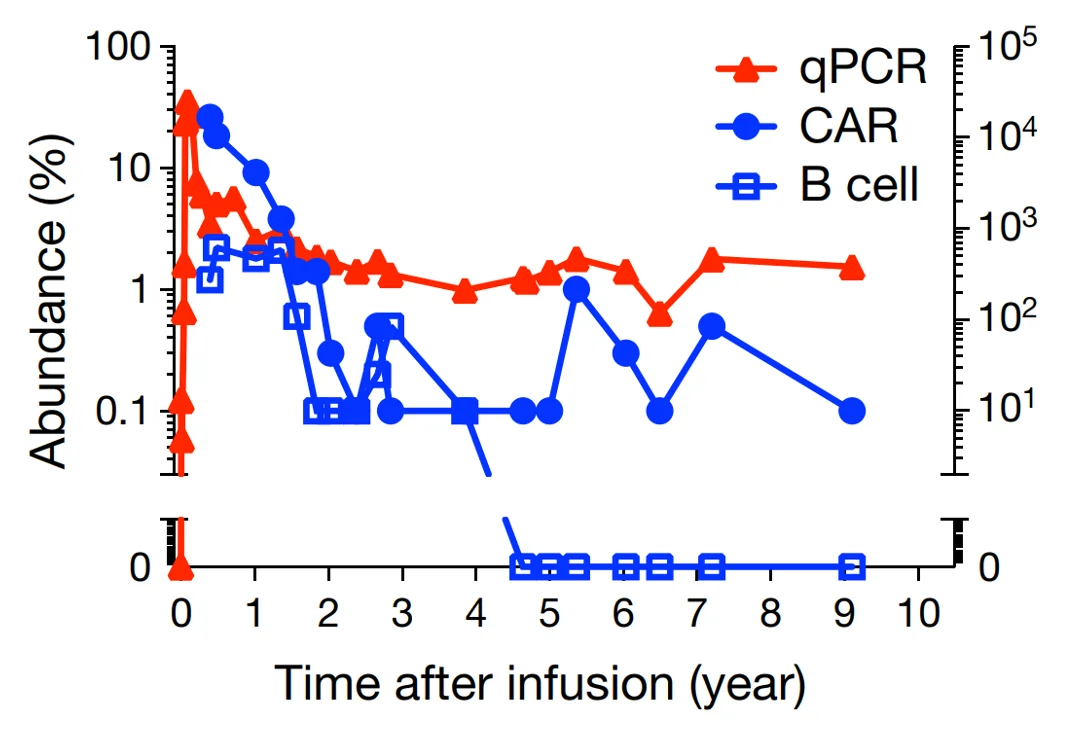
Figure 3. Ten-year monitoring of levels of CAR T-cells (measured via quantitative polymerase chain reaction, qPCR) (red triangles), CAR molecules (blue dots) and B-cells (control, blue squares). Source: Melenhorst et al. (2022), Nature.
Despite the safety concerns, CAR T-cell therapy offers immense benefits with more untapped potential. For one, longitudinal studies have demonstrated that a single infusion of CAR T-cells can maintain anti-cancer activities for up to a decade in patients with leukaemia (Figure 3), contributing to long-term cancer remission and preventing cancer recurrence. While significant advancements in cancer therapy have been made over the decades, leading to improved survival rates, the incidence of cancer recurrence stays persistently high. The advent of CAR T-cell therapy will, hopefully, curtail the longstanding issue of cancer recurrence.
Second, CAR T-cells appear “more natural” to the immune system as the engineered T-cells were derived originally from the patient. This poses no risk of graft-vs-host disease, which occurs when T-cells in the donated stem cells or bone marrow attack the patient’s own tissues. Without immunotherapy like CAR T-cell therapy, patients with severe blood cancer (e.g., leukaemia and lymphoma) are usually treated with stem cell or bone marrow transplants.
Third, CAR T-cell therapy has the potential to overcome tumour heterogeneity, notorious for contributing to treatment resistance. Even with the same type of cancer, the tumours between patients often differ in their metastatic capacity and mutational profile. As a result, a successful cancer therapy for one patient may not necessarily benefit another patient with the same cancer. CAR T-cells, however, can be theoretically engineered to target tumour proteins specific to the individual cancer patient, achieving personalised therapy.
Overall, the innovation of immunotherapy, particularly CAR T-cell therapy, offers a promising method to combat cancer. It achieves long-term remission with fewer complications like graft-vs-host disease. As research continues to refine this therapy, its role in transforming cancer care grows ever more significant, especially where other therapies fall short, offering a future where cancer can be effectively managed or even cured.