ProCAR Therapy: Using Probiotics to Improve Precision and Safety of T-cell Immunotherapy
Introduction to CAR T-cell Therapy
The field of immunotherapy, which harnesses and amplifies the immune system’s inherent anti-cancer capacities, has made striking advancements over the past decade. Innovations such as immune checkpoint inhibitors and chimeric antigen receptor (CAR) T-cell therapy have significantly improved cancer medicine by providing an alternative treatment option for challenging cancer cases, especially advanced and refractory (treatment-resistant) cancers.
Particularly noteworthy is CAR T-cell therapy, a novel and bold strategy that seems to appear out of science fiction. This method involves extracting a patient’s T-cells and genetically engineering them to express chimeric receptors that target antigens on the surface of cancer cells. In biology, chimeric refers to entities that typically exist separately (e.g., engineered receptors), while antigens are molecules that appear foreign to the immune system (e.g., cancer proteins). The engineered receptors are, thus, termed chimeric antigen receptors (CARs). This precision allows the CAR T-cells to attack the targeted cancer cells directly, leveraging the natural abilities of T-cells to destroy foreign cells, such as virus-infected and cancerous cells.
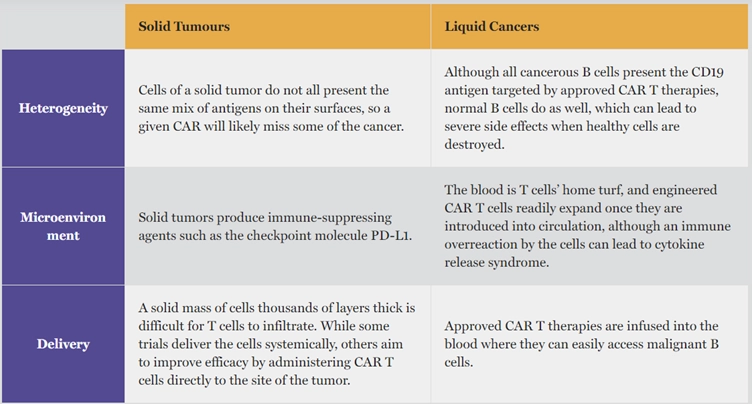
Despite its revolutionary impact, CAR T-cell therapy still grapples with certain obstacles (Figure 1). For instance, pinpointing cancer-specific antigens is challenging in solid tumours. This has limited the success of CAR T-cell therapy mainly to liquid cancers, such as blood and lymphatic cancer, which have more uniformly expressed antigens across their cancer cells. In contrast, the heterogeneity of solid tumours, with a wide variety of mutations and antigen expressions, complicates the identification of specific targets for CAR T-cells.
Moreover, the delivery method of CAR T-cells, involving bloodstream infusion, is inherently more suited to targeting liquid cancers. In such cases, the infused CAR T-cells can circulate and directly engage with the cancerous cells.
Targeting solid cancers, however, is more complex due to the necessity of direct cell-to-cell contact between circulating CAR T-cells and the solid tumour masses. The immunosuppressive microenvironment and irregular vasculature (blood vessels) enveloping solid tumours further undermine the effectiveness of CAR T-cells to penetrate, survive and function within the solid tumour (Figure1).
Figure 1. Factors contributing to the greater efficacy of CAR T-cell therapy in liquid cancers versus solidtumours. Source: Gren and O’Keefe (2019), The Scientist.
These challenges underscore the need for innovative strategies to enhance the effectiveness of CAR T-cell therapy against solid tumours. Emerging research is delving into using probiotics to guide CAR T-cells more effectively to their target solid tumours, which may come with additional perks of lower risks of adverse events. This newsletter ventures into the cutting-edge technology of probiotic-guided CAR T-cell (ProCAR) therapy, and how it builds upon existing achievements of immunotherapy to offer new hope for patients worldwide.
Emergence of ProCAR Therapy
The concept of ProCAR therapy was first pioneered by Vincent et al. from Columbia University’s Department of Biomedical Engineering in New York. Their study was recently published in Science, one of the world’s leading academic journals. Herein, the research team engineered probiotics that could colonise tumours and release antigens to mark the tumour for targeted destruction by CAR T-cells. The study conclusively demonstrated the efficacy of this innovative ProCAR approach in eliminating both liquid and solid cancer cells, representing a notable breakthrough in immunotherapy.
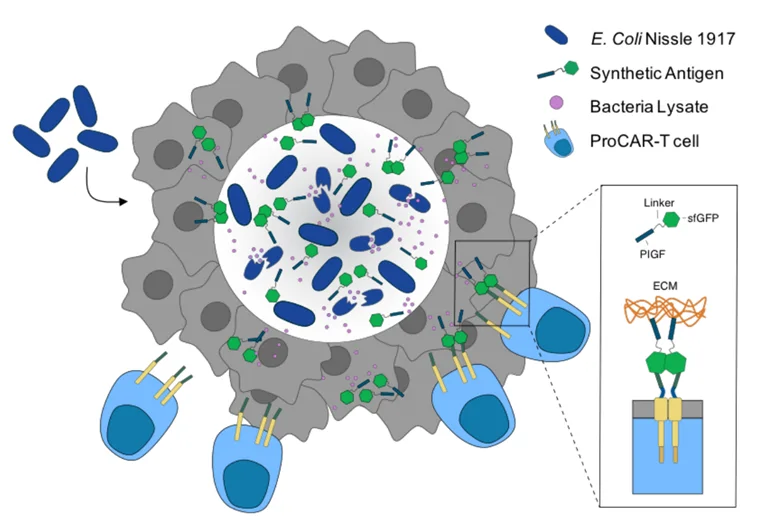
Specifically, the team first engineered a lysis circuit into a well-known probiotic species, Escherichia coli strain Nissle 1917 (EcN). This lysis circuit causes EcN to self-destruct (lyse) and release synthetic antigens upon reaching a certain level of population density in the tumour. These synthetic antigens are tagged with green fluorescent proteins, recognisable by CAR T-cells (Figure2). The CAR T-cells then inject cytotoxic chemicals into the tumour, killing the cancer cells via apoptosis.
EcN was chosen due to its well-documented safety and genetic profile, allowing for ease of genetic manipulation. Moreover, EcN can thrive in low-oxygen conditions typical of tumour environments. The immunosuppressive and nutrient-dense nature of tumour environments further enable the selective proliferation of EcN within them.
Figure 2. Mechanism of probiotic-guided CAR T-cell (ProCAR) therapy. Engineered E. coli Nissle 1917 (dark blue) specifically colonises the solid tumour and releases synthetic antigens (green) through its programmed circuit lysis. Such antigens are designed to adhere to the extracellular matrix (ECM, brown) components of the tumour, as well as to direct CAR T-cells (blue) to the tumour site (grey). Source: Preprint version of Vincent et al. (2023), Science.
The synthetic antigens also contain a heparin-binding domain, which adheres to the rich collagen and fibronectin components of the tumour’s extracellular matrix (ECM). Compared to liquid cancers, solid tumours build denser ECM to strengthen tissue rigidity, which helps enhance tumour aggressiveness and acts as a barrier against the immune system and therapeutic drugs. As a result, this EcN design confines the antigens within the ECM-dense solid tumour, reducing the risk of off-target toxicity. Therefore, the cornerstone of this approach is the bacteria’s ability to grow exclusively in the harsh living conditions of solid tumours and torelease antigens tagging the tumour for cytotoxic attack by CAR T-cells (Figure2).
Current Status and Future Potential
Vincent et al. also investigated the efficacy of their ProCAR technology in a xenograft mouse model, where cancer cells from human patients with blood or breast cancer were implanted into the mice. This model helps study human diseases in living organisms in controlled laboratory settings. They then injected either engineered EcN, non-engineered EcN or placebo into the xenograft mice, followed by CAR T-cell infusion 48 hours later.
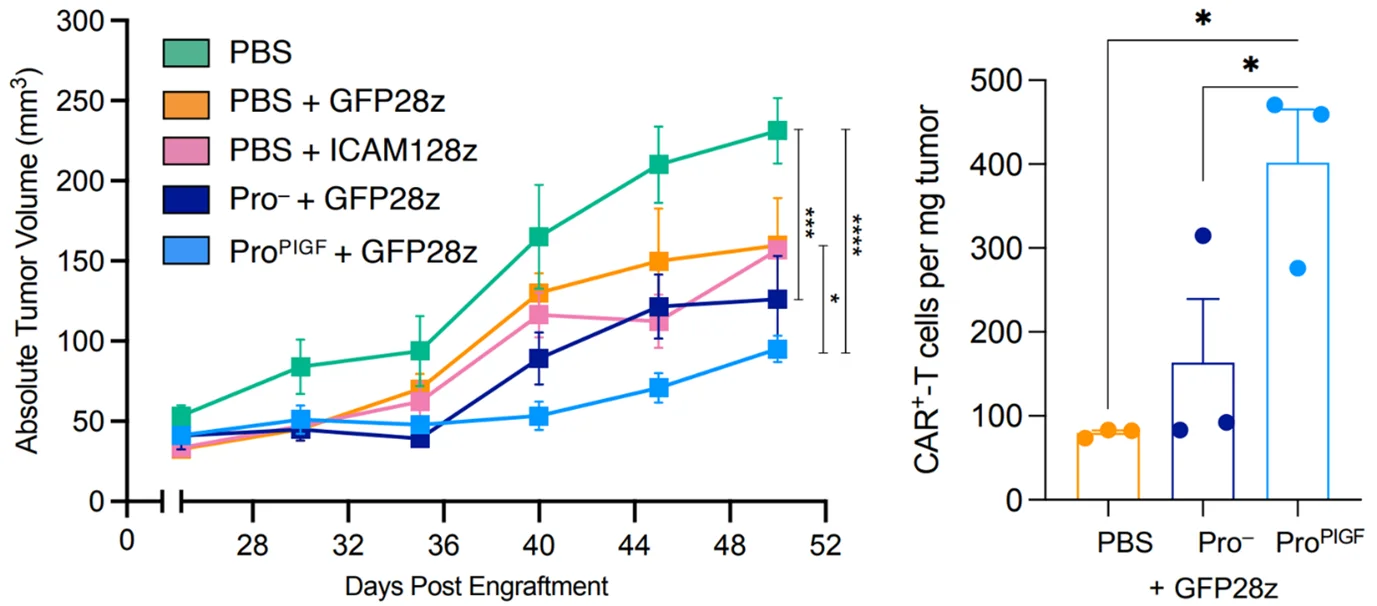
Results revealed that mice administered with the engineered EcN showed the most pronounced tumour shrinkage and the highest infiltration of CAR T-cells into the targeted tumour site (Figure 3). These mice also maintained normal body weight throughout the study, indicating the absence of adverse effects on their overall health. Crucially, the growth of EcN was confined to the tumour and absent in healthy organs, which substantiates the tumour-specific colonisation of EcN. Additionally, EcN administration provoked broader immune responses against the tumour beyond just T-cells, showing that the inherent immunogenic nature of bacteria further contributes to the anti-tumour effects of this therapy.
While this study only examined the efficacy and safety of ProCAR therapy in mice implanted with human cancer cells, it convincingly showcased its potential to overcome the challenges of using CART-cell therapy for solid tumours. Moreover, the enhanced tumour specificity of ProCAR therapy could mitigate the risk of off-target toxicity, i.e., unintended damage to non-cancerous cells. This is a serious safety issue with the current CAR T-cell therapy, which can cause neurotoxicity when the targeted antigens on cancerous cells are also present on healthy brain cells. As a result, rare instances of infiltration of CAR T-cells into the brain, causing symptoms such as delirium and seizures, have been documented in clinical trials.
Figure 3. The efficacy of probiotic-guided CAR T-cell therapy in a mouse xenograft model with implanted human breast cancer cells. The mice received either engineered probiotic (ProPIGF),non-engineered probiotic (Pro—) or placebo (PBS) before beingadministered with green fluorescent protein-targeting CAR T-cells (GFP28z) 48hours later. Left: The lowest tumour volume was observed in mice treated withengineered probiotic. Right: The highest number of tumour-localised CAR T-cellswas observed in mice treated with engineered probiotics. Source: Preprint version of Vincent et al. (2023), Science.
Overall, ProCAR stands as a promising advancement in the field of immunotherapy, offering a safer and more precise version of CAR T-cell therapy. Looking ahead, the research team at Columbia University is actively preparing for clinical trials to test ProCAR therapy in humans. They are hopeful that, through further refinements, this therapy could emerge as a universal approach for treating all or most varieties of solid tumours. As ProCAR therapy bypasses the need to identify specific tumour antigens, CAR T-cell products need not be tailored for every type and case of cancer, which greatly streamlines the treatment process.
CAR T-cell therapy has already shown remarkable success in treating advanced or treatment-resistant liquid cancers. With continued research and development, ProCAR therapy holds the potential to extend this success to patients battling difficult-to-treat solid tumours, offering new hope where treatment options were previously limited.