How Liquid Biopsy Overcomes Risks and Limitations of Traditional Tissue Biopsy
from Integrative Cancer Care Newsletter, April 2023
Background
Scientists initially thought cancerous cells were localised in the tumour only, until 1869 when circulating tumour cells (CTCs) were discovered in the blood of a patient with metastatic cancer. It took another century for scientists to understand that some cancer cells often dislodge from the primary tumour as CTCs into the blood to establish another cancerous tumour elsewhere. This is the ‘seed and soil’ hypothesis of metastasis or cancer spread. When scientists realised this, the potential of using liquid biopsy to detect CTCs for diagnosing cancer came to light.
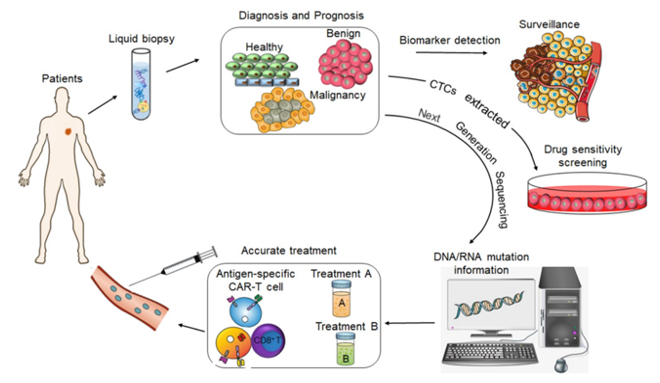
In recent years, the clinical applications of liquid biopsy have advanced rapidly. In this coming generation, liquid biopsy may revolutionise cancer detection and monitoring, paying the way for more effective treatments and, ultimately, precision cancer medicine (Figure 1).
Figure 1. The value of liquid biopsy in tumour diagnosis and precision medicine. Source: Wu et al. (2020) under CC BY 4.0.
Benefits Over Traditional Biopsy
Traditional tissue biopsy typically uses a needle to penetrate the tumour to extract a small piece of sample for the analysis of cancer biomarkers. Despite its necessity to verify cancer diagnosis, tissue biopsy is a minor surgery with multiple drawbacks:
- Invasive and painful
- Costly
- Time-consuming
- Impractical for long-term monitoring
- Incapable of capturing the profile of heterogeneous tumour, and
- Risky (i.e., may dislodge cancer cells from the tumour and cause other complications such as infection and bleeding)
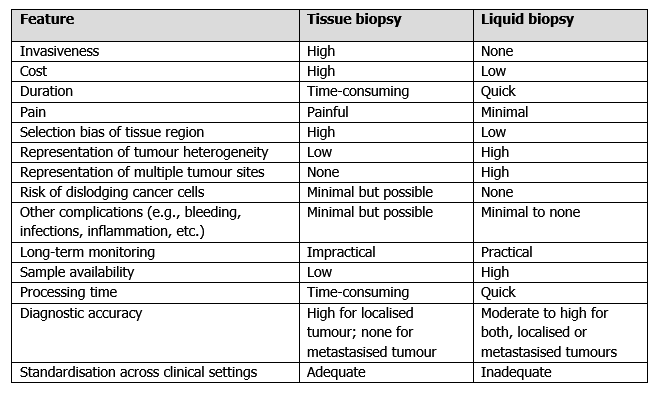
Liquid biopsy circumvents most, if not all, limitations of tissue biopsy (Table 1). As liquid biopsy is akin to routine blood testing, it is non-invasive, less painful, cheaper, more time-saving and more practical for longitudinal monitoring compared to tissue biopsy. About the more complicated aspects, liquid biopsy also captures tumour heterogeneity more accurately and poses fewer risks of cancer cell dissemination, infection, and bleeding than tissue biopsy; let us see why.
Tissue biopsy only analyses a tiny portion of the tumour, which may not fully reflect the tumour’s cancerous nature. Most tumours are highly diverse, where cancer cells within the same tumour exhibit different behaviours in gene expression, metabolism, and metastatic capacity. As a result, region selection bias often happens with tissue biopsy, which may underestimate the cancerous capacity of tumours. This may lead to poor choice of treatments and contribute to the prevailing problem of treatment resistance and cancer recurrence in oncology.
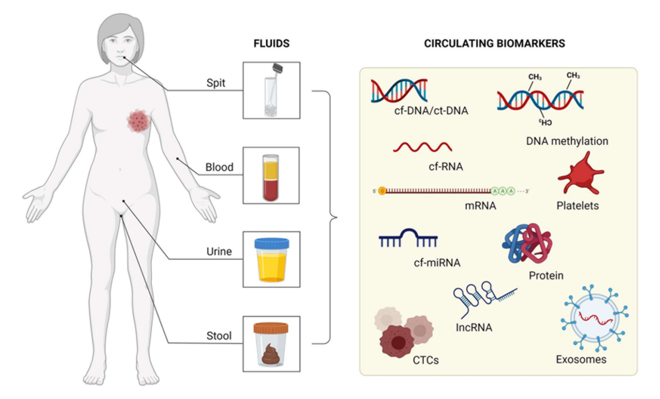
In contrast, samples collected via serial liquid biopsy better represent the overall cancer cells from the tumour, thus reflecting tumour heterogeneity more accurately. Liquid biopsy also examines multiple tumours concurrently, given that cancer markers from all tumour sites are released into the blood. The term tumour circulome was coined to reflect the fact that cancer-derived markers (e.g., CTCs, circulating tumour DNA, and tumour-derived proteins and extracellular vesicles) are leaked into the blood, which liquid biopsy can capture (Figure 2).
Numerous studies have championed the use of liquid biopsy over traditional tissue biopsy in cancer care. For example, liquid biopsy CTCs analyses are better apt at detecting epigenetic changes, which provide important information on cancer evolution, compared to tissue biopsy in patients with colorectal, prostate or breast cancer. Certain cancer mutations missed from tissue biopsy have been successfully detected in circulating tumour DNA (ctDNA) sampled via liquid biopsy. Further studies showed that ctDNA-based liquid biopsy detected and predicted cancer development, progression and recurrence early in ovarian, lung, breast, prostate and colorectal cancer patients, which guided therapy modification and prevented overtreatment. While CTCs and ctDNA are the only liquid biopsy biomarkers the FDA approved, tumour-derived RNA, proteins and exosomes, epitope detection in monocytes, and tumour-educated platelets are more recent liquid biopsy biomarkers whose full potential remains to be unearthed (Figure 2).
Figure 2. Fluids that can be used as circulating biomarkers in liquid biopsy. Although blood is most commonly used in liquid biopsy, other choices of biofluids include spit, urine and stool. Source: Freitas et al. (2022) under CC BY 4.0.
Tissue biopsy risks dislodging cancer cells from the penetrated tumour. A few other scientists and I warned about this risk decades ago but were largely ignored by the medical community. As more research gets done, disseminated cancer cells are now a small but acknowledged risk of tissue biopsy. Unlike healthy tissues, tumours are unstable entities with weak cell-to-cell adhesion but rapid cell proliferation rates. Using biopsy needles to disrupt tumours may break free some cancer cells, which may facilitate cancer spread or recurrence in rare instances. As liquid biopsy does not disturb the tumour directly, the risk of dislodged cancer cells from the tumour is non-existent.
Besides, tissue biopsy is a minor form of surgery with risks of infections, internal bleeding and tissue inflammation. Liquid biopsy only entails needle penetration at the vein, which would not cause these complications. For cancer monitoring, repeated tissue biopsies are required, which is impractical given the costs and risks involved with every biopsy. Tissue biopsy also faces low sample availability and longer processing time – limitations not seen with liquid biopsy. So, liquid biopsy is a highly valuable tool for the long-term monitoring of cancer and treatment response, given that cancer is a recurrent and chronic disease.
Current Challenges and Future Potential
Owing to its novelty, liquid biopsy still has a few challenges to overcome. One drawback is its lower accuracy at detecting certain cancers compared to tissue biopsy, which penetrates and samples the tumour directly. Even so, the added information from liquid biopsy offers insights into tumour characteristics not always obtainable via tissue biopsy, such as tumour heterogeneity and metastasis capacity (Table 1). Another limitation of liquid biopsy is the lack of standardisation of sample collection, purification and analyses in clinical settings. However, efforts are being made to establish cost-effective, rapid, and automated liquid biopsy analyses.
Despite that, it is no exaggeration that liquid biopsy holds immense potential to initiate a paradigm shift in cancer detection and monitoring. With further research, liquid biopsy may very well replace tissue biopsy as the standard diagnostic technique, allowing patients and physicians to get diagnostic information about the cancer without facing the risks of tissue biopsy (Table 1).
Despite technological advancements, cancer is still a leading cause of death, poor life quality and financial burden worldwide. Effective cancer medicine, thus, remains an utmost priority for the medical and scientific communities to achieve. By capitalising on the potential of liquid biopsy, cancer medicine can be revolutionised, and effective personalised cancer care can become a reality. Unfortunately, tissue biopsy has been unsuccessful in this regard. As Albert Einstein said, “We cannot solve our problems with the same thinking we used when we created them”.