Introduction
In 1907, Dr Charles Childe, a British surgeon, advocated the importance of early cancer detection in his book “The Control of a Scourge, Or How Cancer Is Curable.” He posited that most cancers could be treated effectively if caught early. While his work had limited influence in the British medical system, it inspired the American Medical Association to promote periodic health checks to identify cancer early, introducing “screening” into the medical language in the 1920s.
During that period, clinical trials were not widely practised. As a result, medicine relied more on professional judgements and anecdotal case reports. However, as clinical trials evolved to become the benchmark for shaping medical guidelines, the effectiveness of cancer screening tests has come under scrutiny, prompting a re-evaluation of their benefits in contemporary healthcare.
For example, a 2023 meta-analysis of 18 clinical trials found that nearly all of the most common cancer screening tests do not prolong life expectancy over the long run. This meta-analysis, involving 10-15 years of data of over two million patients, revealed that only sigmoidoscopy used for colorectal cancer screening significantly extended life expectancy by 110 days. Conversely, the other standard screening tests (e.g., computed tomography for lung cancer, colonoscopy for colon cancer and mammography for breast cancer) did not influence life expectancy.
Mammography, in particular, has been at the centre of intense debate concerning its risk-benefit ratio. This newsletter aims to delve into the real effectiveness and safety of mammography in breast cancer screening and explore potential strategies for enhancing its utility.
Decoding the Effectiveness of Mammography
Mammography is not entirely useless, however. Regarding the meta-analytical findings, critics argued that while screenings may not substantially alter overall life expectancy, they can prevent early death from cancer. For instance, cancer screening may not extend life expectancy from 80 to 90 years, but it can thwart an untimely demise at 65 years and allow lifespan to reach the expected 80 years. Therefore, whether mammography is effective or not depends on the metric used.
Mammography is useful in catching early breast cancer, even before symptoms surface. One study reported that mammography-screened breast cancer had a 10-year survival rate of 80%, compared to 63% for cancer cases detected through symptom onset. The stage of cancer often determines the intensity of treatment required, with advanced stages necessitating more aggressive therapies. Research showed that unscreened women are more than twice as likely to need intensive treatments, such as chemotherapy and surgery, compared to those who undergo regular screenings. Hence, such findings illustrate that although mammography may not be universally beneficial, it makes a significant difference to a subset of women with hidden, underlying breast cancer.
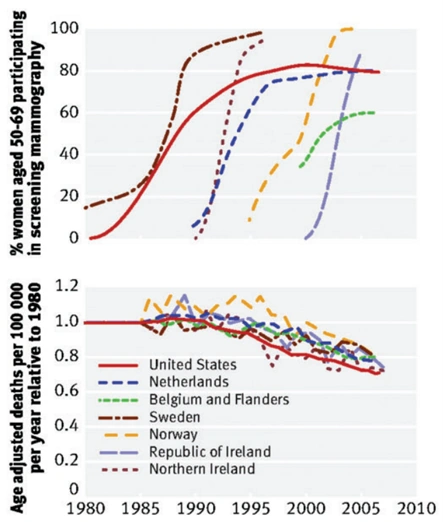
However, one caveat is the high number needed to screen (NNS) metric, i.e., how many women need to be screened to prevent one death. A systematic review puts the NNS of mammography at 2,449, 1,503 and 1,159 for women aged 40-49, 50-59 and 60-69 years, respectively. Thus, at least a thousand women have to undergo mammography to save one woman from breast cancer. This also explains why the implementation of regular mammography screening has not correlated with a reduction in breast cancer mortality at the national level (Figure 1). Instead, the decline in breast cancer mortality began around 1990 across countries, concurrent with the introduction of more effective cancer therapies. To this end, the Swiss Medical Board has recommended terminating mammography screening altogether due to insufficient benefits to justify its risks and harms.
Figure 1. The implementation of regular mammography screening did not correlate with the national mortality rates of breast cancer. Top: Percentages of women participating in regular mammography screening in each country. Bottom: Changes in breast cancer mortality rates in every country. Source: Gøtzscheet (2015), Journal of the Royal Society of Medicine.
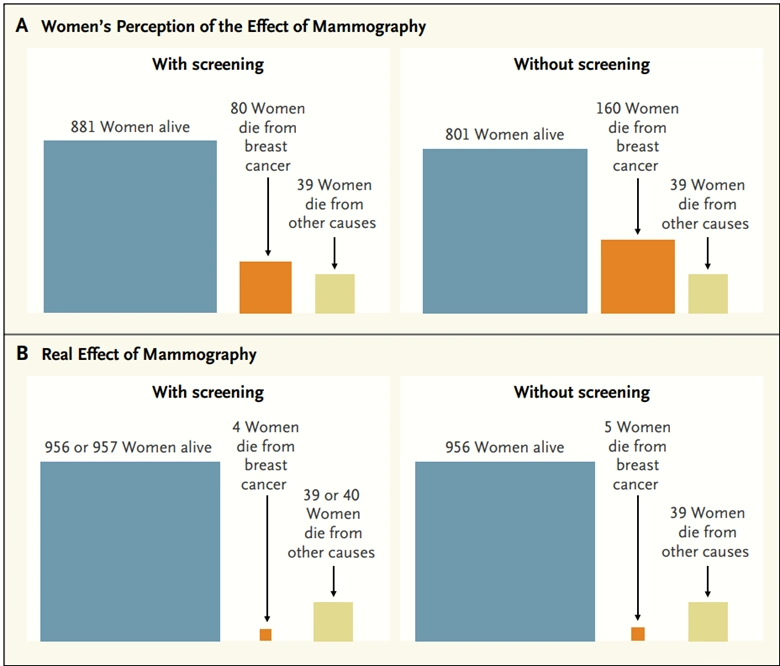
The Swiss Medical Board also pointed out they were disturbed that most women drastically overestimate the benefit of mammography. A survey study conducted across the U.S., the U.K., Italy and Switzerland revealed about 70% of women believed that mammography screening, at the very least, cuts breast cancer mortality by 50% and prevents 10 deaths per 1,000 women. The actual NNS metric, however, clearly indicates otherwise (Figure 2).
Notably, the survey did not examine the women’s awareness of the potential negative effects of mammography, such as misdiagnoses and radiation exposure, further suggesting that women may hold unrealistic expectations of the benefit-risk ratio of mammography screening.
Figure 2. Perception of women on the benefits of mammography screening on breast cancer mortality compared to the actual benefit. Source: Biller-Andorno and Jüni (2014), New England Journal of Medicine.
Indeed, the accuracy of mammography is not perfect, standing at about 85-90% accuracy. While this appears high, it also means the false positive (i.e., detecting cancer when there is none) rate is about 10%. Subjecting all women to mammography would, thus, result in 10% of the population being misdiagnosed with non-existent breast cancer. With 10 years of annual mammography screenings, the chance of false positives escalates to 50-60%.
Such misdiagnoses can cause unnecessary anxiety and lead to medical interventions that might not only be futile but pose additional risks and costs. For example, in a group of 1,000 women aged 50 undergoing annual mammography for a decade, between 0.3 and 3.2 women might be saved from breast cancer death. However, this practice leads to 490-670 women receiving at least one false positive diagnosis, with 70-100 and 3-14 of them undergoing unneeded biopsies and treatments (e.g., radiation, surgery, or chemotherapy), respectively. As a result, the economic burden of regular mammography screening is estimated to cost billions of dollars per year in the U.S. alone.
Decoding the Safety of Mammography
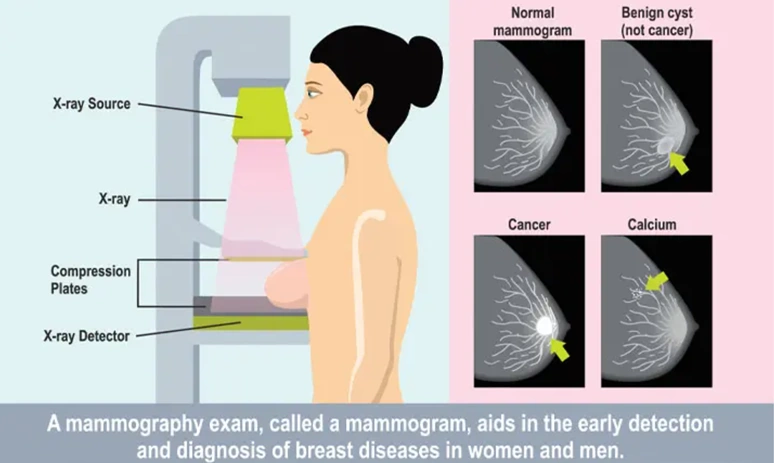
Mammography works by using low doses of ionising radiation in the form of X-rays to create detailed images of the breast tissue (Figure 3). These images can then be examined for signs of cancer, such as lumps or microcalcifications. However, excessive radiation exposure causes DNA damage and cancer. As such, radiation-related cancer is also a small but possible risk of mammography. This risk is especially concerning for individuals with genetic predispositions that impair DNA repair activities. For women aged 40 years and older, research estimated that mammography saves 36.5 lives for every life lost to radiation exposure.
Samuel Epstein, MD, was among the earliest physicians to scrutinise the risks of mammography screening publicly in the 1990s. Dr Epstein highlighted the standard procedure of capturing four mammograms for each breast yearly exposes women to around 1 rad (radiation absorbed dose), which may be sufficient to raise the risk of breast cancer by 0.8%. This level of exposure is also 1,000 times higher than the radiation from a single chest X-ray. Notably, mass screening using chest X-rays to identify early cases of lung cancer and tuberculosis was discontinued in many countries due to its tendency to induce more cancer than it detected.
Figure 3. How mammography works. Source: Mount Elizabeth Hospital (2021).
Mammography is also not a painless experience. To obtain precise images, the mammography machine needs to compress the breast. Systematic reviews indicated that 77% and 28% of women experienced pain and considerable pain during mammography, and pain deterred almost 50% of women from attending the subsequent mammography screening. More concerningly, Dr Epstein cautioned that such compression may rupture small blood vessels in or around an undetected breast cancer. This could potentially trigger the dissemination of cancer cells to the bloodstream, which may result in a heightened risk of metastatic cancer.
Strategies to Circumvent the Risks of Mammography
Despite the effectiveness and safety concerns of mammography, discarding this technology is not a practical approach. Mammography screening still benefits the small subset of women at risk of developing breast cancer and is crucial in aiding breast cancer diagnoses. Therefore, research efforts should focus on exploring individualised approaches to screening, methods to complement the utility of mammography and even new technology to replace mammography.
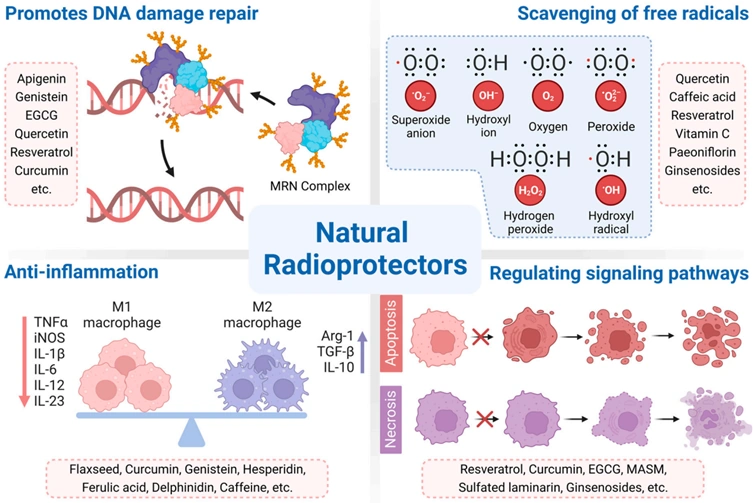
Individualised screening should entail screening only those at risk of developing breast cancer, such as having a family history of breast cancer, personal history of radiotherapy to the chest or certain genetic risk factors. Complementary medicine, aimed at minimising oxidative stress and DNA damage from ionising radiation or X-rays, would also be helpful. The Pfeifer Protocol is a notable example, utilising plant-derived compounds with robust bioactive effects, including anti-cancer, antioxidant and DNA repair properties. A comprehensive 2023 review paper underscored the radioprotective mechanisms of several plant compounds (Figure 3), most of which are also included in the Pfeifer Protocol, such as:
- Resveratrol, a polyphenol found in grapes and berries, has been found to protect cells from radiation-induced DNA damage. Under oxidative stress, an enzyme called tyrosyl-tRNA synthetase (TyrRS) moves into the cell’s nucleus to safeguard the DNA. Resveratrol can enhance the activity of TyrRS, allowing it to enter the nucleus faster to prevent DNA damage more effectively.
- Curcumin, a polyphenol from turmeric, can facilitate the repair of double-strand breaks in DNA, which are considered the worst form of DNA damage. Curcumin achieved this via multifaceted mechanisms, such as activating DNA repair enzymes and reducing oxidative stress and inflammation.
- Quercetin, a flavonoid abundant in fruits and vegetables, has also been shown to suppress DNA double-strand breaks in cells exposed to X-rays by upregulating the expression of Bmi-1, an indispensable protein involved in wound healing and DNA reparation.
Figure 4. A summary of the radioprotective mechanisms of certain plant compounds, including repairing DNA damage, scavenging free radicals to reduce oxidative stress, reducing inflammation and regulating cell signalling. Source: Zhang et al. (2023), Cancers.
Alternatively, other methods can be considered for early detection of breast cancer, such as thermography, magnetic resonance imaging (MRI) and liquid biopsy. These methods, however, are less widely used than mammography for several reasons, such as sub-par accuracy, high costs and unstandardised protocols, therefore necessitating further developments.
- First, thermography uses infrared cameras to capture heat patterns and blood flow in breast tissues, which aims to identify abnormal temperature variations indicative of cancer. The Food and Drug Administration (FDA) has approved thermography as an adjunctive tool, which means it should only be used alongside a primary method of detecting breast cancer. However, efforts are being made to enhance the accuracy of thermography, which offers several benefits over mammography, such as lower costs and no radiation.
- Second, MRI uses magnetic fields and radio waves to create detailed images of the breast tissue to identify abnormalities or tumours. However, it faces challenges such as high cost and limited availability compared to mammography. Nevertheless, MRI is particularly advantageous in detecting breast cancer in younger women with denser breast tissue or those with breast implants, where mammography’s accuracy diminishes. Additional perks of MRI include no radiation exposure and no physical compression.
- Third, liquid biopsy for breast cancer detection works by analysing blood samples for circulating tumour cells or cancer cell DNA. While this method offers a non-invasive method to detect and monitor cancer, its limitations include lower accuracy for localised tumours and the lack of standardisation in how samples are collected and analysed. Regardless, initiatives are ongoing to develop automated liquid biopsy analysis with improved accuracy.
Overall, the ability of mammography to detect early breast cancer is undoubtedly crucial, yet concerns about accuracy, discomfort and radiation exposure call for more nuanced approaches. This includes personalising screening protocols and implementing complementary strategies to mitigate the potential negative effects of mammography. Furthermore, there is a pressing need to advance the development of promising alternative detection methods. The goal is to evolve beyond relying solely on mammography as the standard approach for breast cancer detection, thereby enhancing medical practices in this field.